The electro-chemo-mechanical coupling at the solid-liquid interfaces and its applications to electrocatalysis
-
摘要: 目前许多新型高效金属催化剂在设计制备中都考虑到表面力学因素, 例如层状结构、核壳结构等, 其表面高活性原子受到不同程度的应变作用. 应变可直接改变金属的能带带隙, 对催化剂表面的电化学反应产生显著影响, 是一种有效提升材料催化活性的新思路和制备高性能催化剂的新途径, 因此受到了科研工作者的广泛关注. 传统的材料应变工程手段存在着活性物质层的应变值难以精确定量, 并缺少实时调控以及制备工艺繁琐等难题, 导致应变与电催化活性相关性规律识别方面的理论和实验研究进展缓慢. 相比于传统的材料手段, 交变载荷产生的应变具有幅值和频率的可变性以及连续的调控性, 在实验中可以完全排除噪声、缺陷、空位、基底效应等其他外部或材料本征的影响因素. 该综述从经典固液界面热力学表述出发, 简要介绍了电催化体系中的力−电−化学耦合效应, 归纳总结目前电催化体系中应变施加的实验手段和分析方法, 并基于目前相关研究着重讨论在交变载荷作用下应变对金属表面电催化反应的作用机理, 最后从力学角度展望了表面力学在电催化体系中的研究重点及发展趋势.
-
关键词:
- 力−电−化学耦合 /
- 电化学麦克斯韦关系式 /
- 交变载荷 /
- 应变工程 /
- 电催化动力学模型
Abstract: Many advanced catalysts have considered the positive effect of surface mechanics during their design and preparation, in which the high active atoms on the surface are under different strain states. Strain can directly change the bandgap of a metal, which has a significant impact on the electrochemical reaction that occurred at the electrocatalyst surface. It is a new idea and effective strategy to improve the electrocatalytic activity of materials. Traditional strain engineering based on material strategies is difficult to accurately quantify the strain value of an active layer, which results in the unclear recognition of the relation between strain and electrocatalytic activity. The strain induced by the alternating load has the advantages of tunable amplitude and frequency as well as continuous modulation. From the classical thermodynamic of solid-liquid interface, this review briefly introduces the electro-chemo-mechanical coupling in electrocatalytic systems, and summarizes the experimental methods and the analysis methods in use for studying the effect of strain on electrocatalytic reactivity. It is also discussed in detail on the mechanism of strain on the electrocatalytic reaction at the metal surface under alternating load. Finally, the development and application of surface mechanics in electrochemical systems are prospected from the perspective of mechanics. -
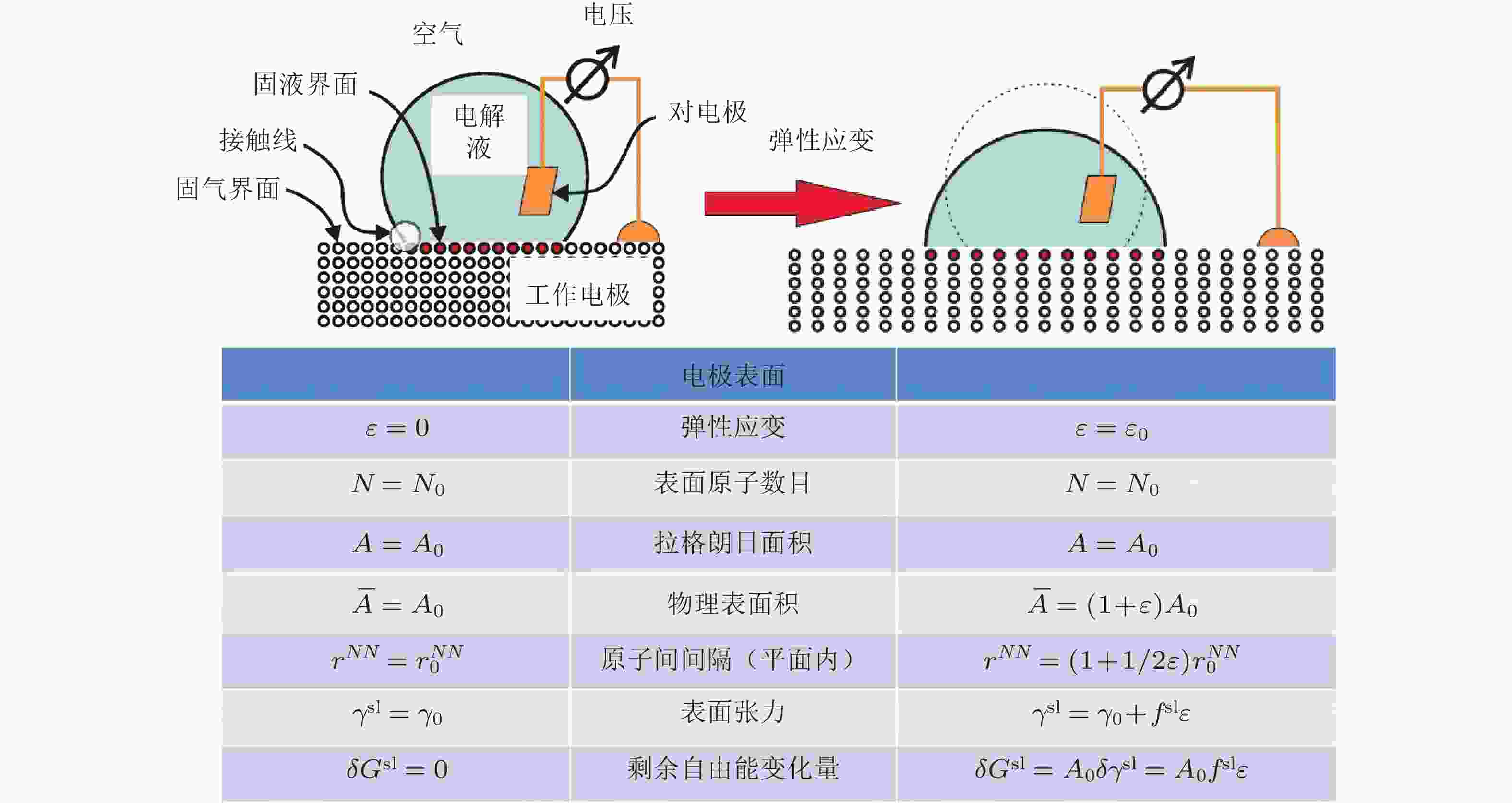
图 1 在电极电势保持恒定时, 对电极施加弹性应变(ε)作用(图1右侧), 金属材料面内原子间距从
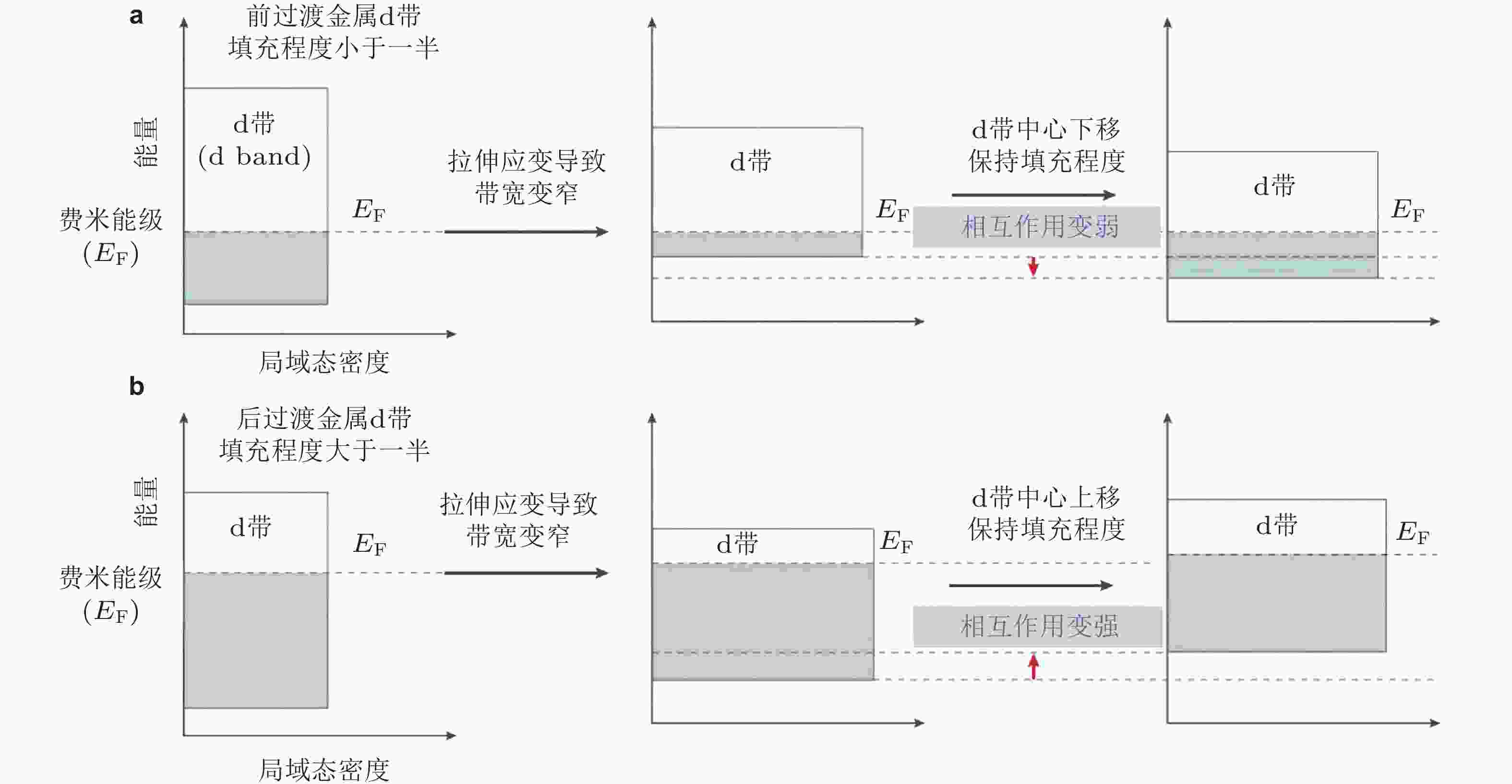
${r}_{0}^{{N}{N}}$ 增大到$ {r}_{0}^{{N}{N}}\left(1+\dfrac{1}{2} \varepsilon \right) $ , 进而增大电极物理表面积($ \overline{A} $ ). 另外,拉格朗日面积A和表面原子数目N保持不变. 系统吉布斯自由能的变化量与表面应力 (f sl) 成比例, 即${\text{δ }}{G^{{\rm{sl}}}} = {A_0}{f^{{\text{sl}}}}\varepsilon$ (Weissmüller 2013)图 2 d带模型理论: 对于前过渡金属(a)和后过渡金属(b)拉伸应变对其d带位置的影响. 拉伸应变都会使前过渡和后过渡金属的d带宽度变窄, 为了保持d带填充程度(即电荷守恒), 前过渡金属d带中心将下移, 而后过渡金属d带中心上移 (Luo & Guo 2017)
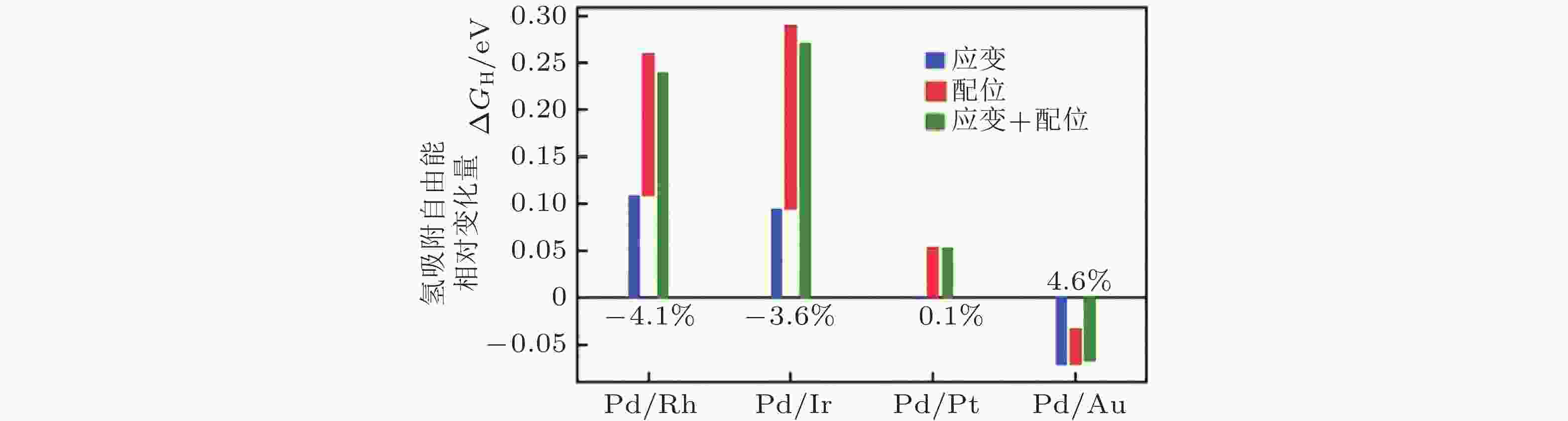
图 3 应变效应和配位效应对氢在Pd表面吸附自由能的影响 (Maark & Peterson 2014)
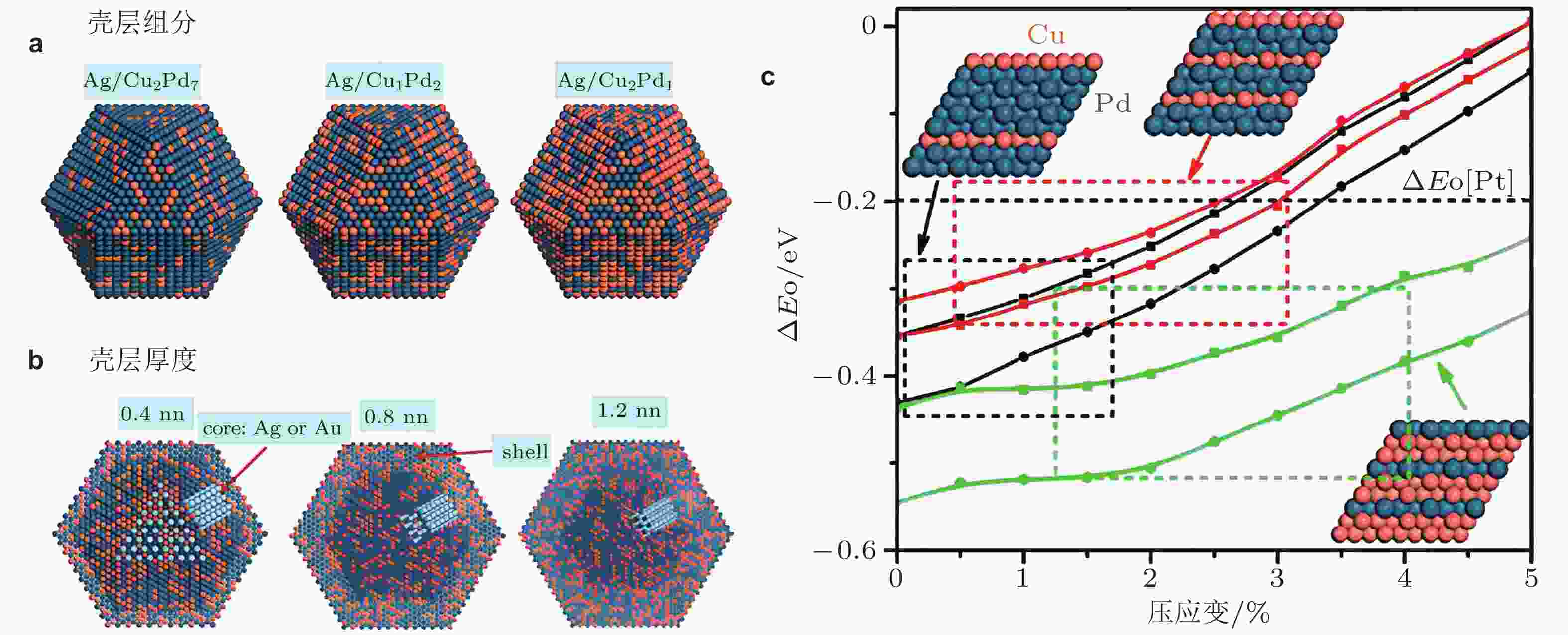
图 4 以不同铜钯组分(a)和厚度(b)为壳层, 银(或金)为核体的核壳结构材料, 氧相对结合能(ΔEO)随应变的变化关系(c). 黑色、红色和绿色的曲线分别代表壳层组分Cu∶Pd的原子比值为2∶7、1∶2和2∶1. 相同颜色的不同曲线对应于氧在不同的面中心立方空心吸附. Pt(111)表面上的ΔEO用水平虚线表示 (Guo et al. 2014)
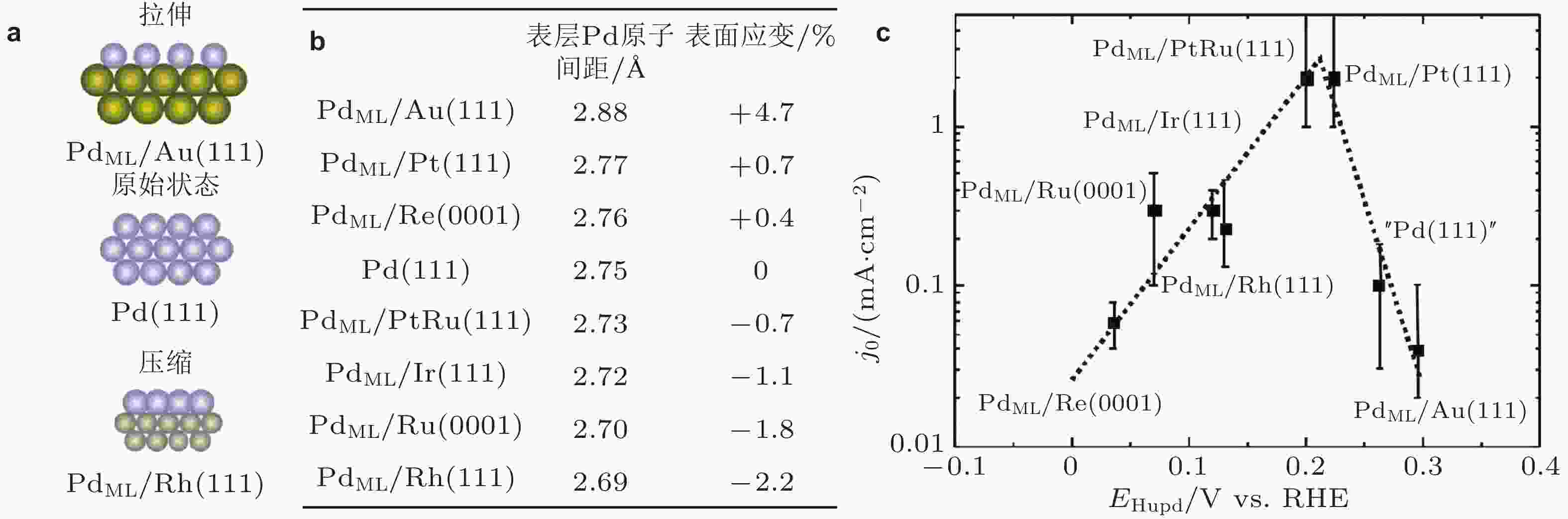
图 5 (a)金属钯Pd(111)以及外延生长在不同基底上的钯单原子层(PdML)晶格关系图, (b)不同双相层状结构表层Pd原子间隔(1 Å = 0.1 nm), (c) Pd(111)以及PdML的析氢反应性能 (Kibler et al. 2005, Kibler 2008)
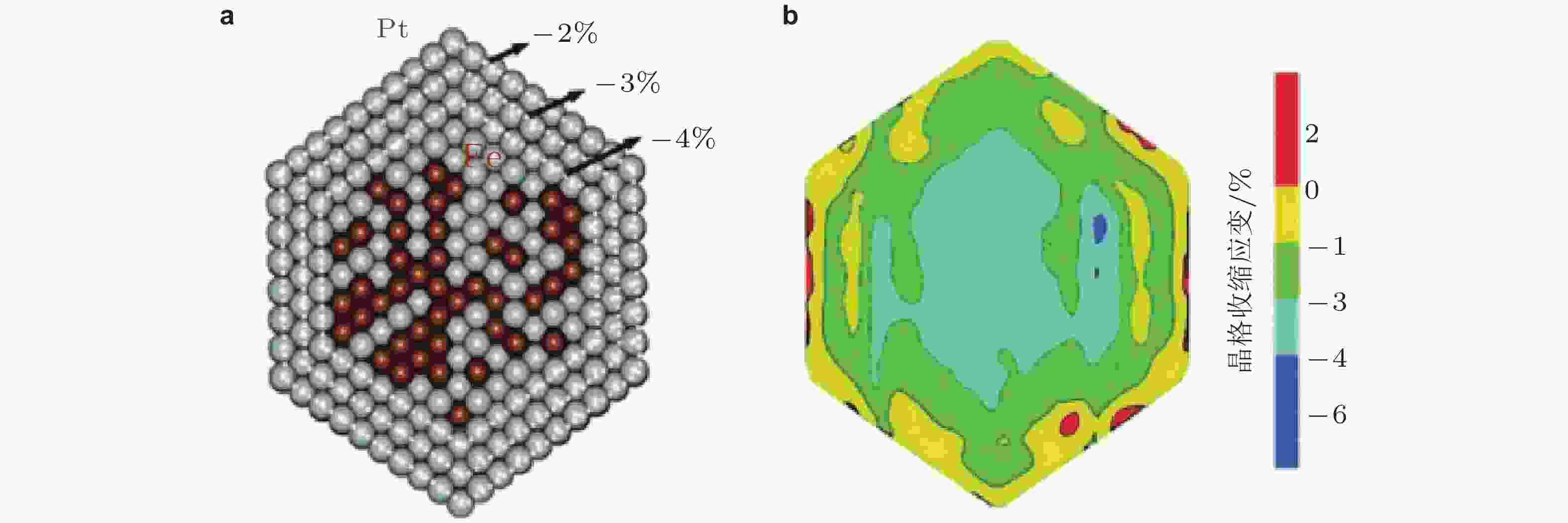
图 6 (a)以Pt50Fe50合金为核Pt为壳的结构模型, 压应变梯度从−4% ~ −2% (相对Pt体相材料); (b)晶格收缩应变图 (Seh et al. 2017)
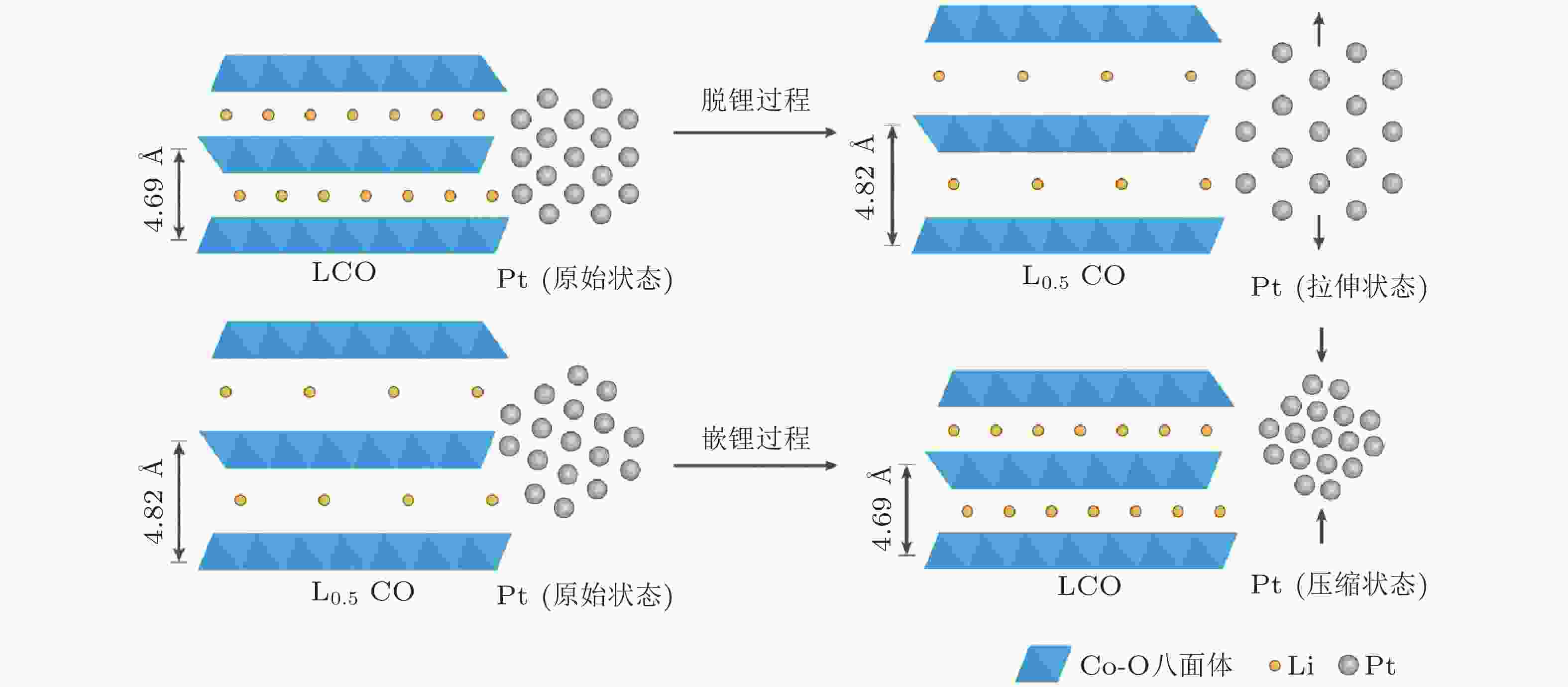
图 7 基于锂离子电池电极材料LiCoO2(LCO)的充放电来改变催化活性物质Pt纳米颗粒的晶格应变 (Wang et al. 2016)
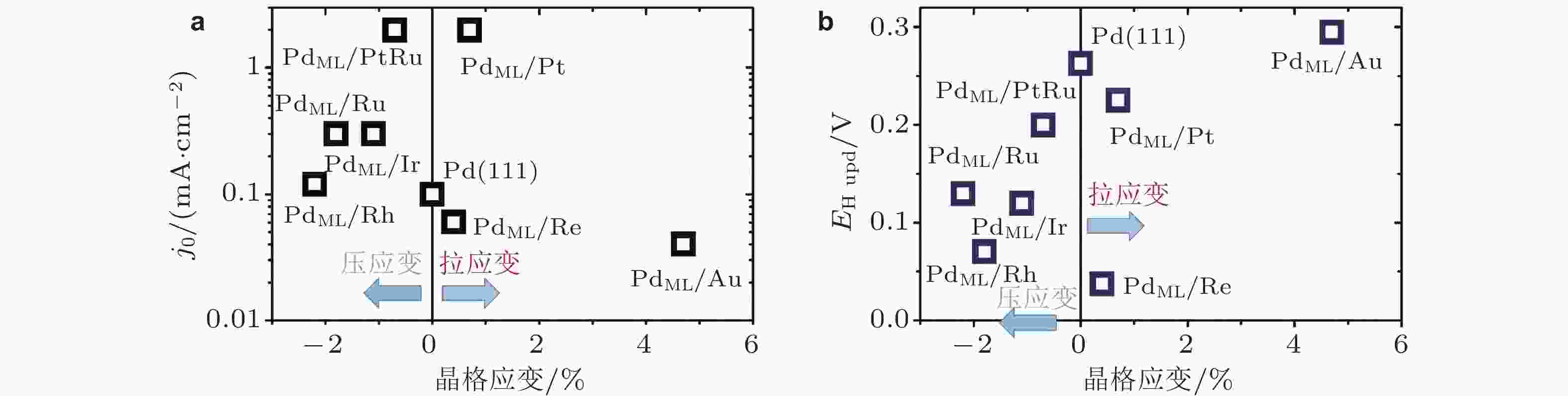
图 8 (a)析氢反应交换电流密度(j0)与晶格应变的关联性, (b)氢吸附电势(EH upd)与晶格应变的关联性 (图中数据来自于图5)
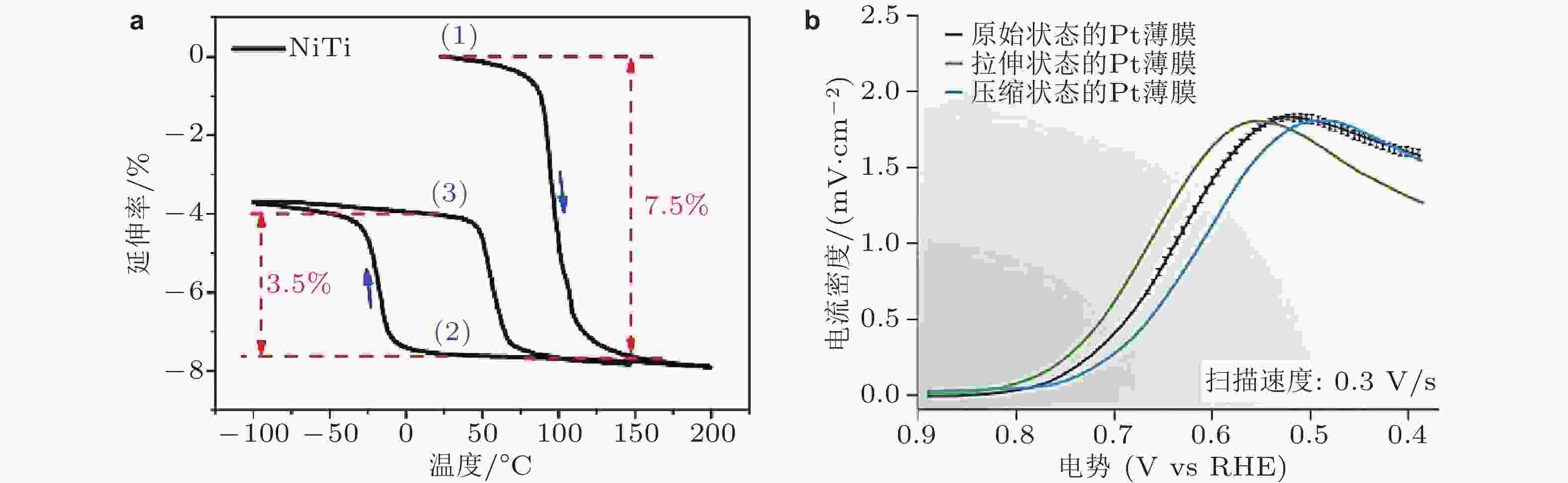
图 9 (a)预变形的NiTi衬底的相变行为, (b)表面不同应变状态下Pt薄膜的电化学性能 (Du et al. 2015)
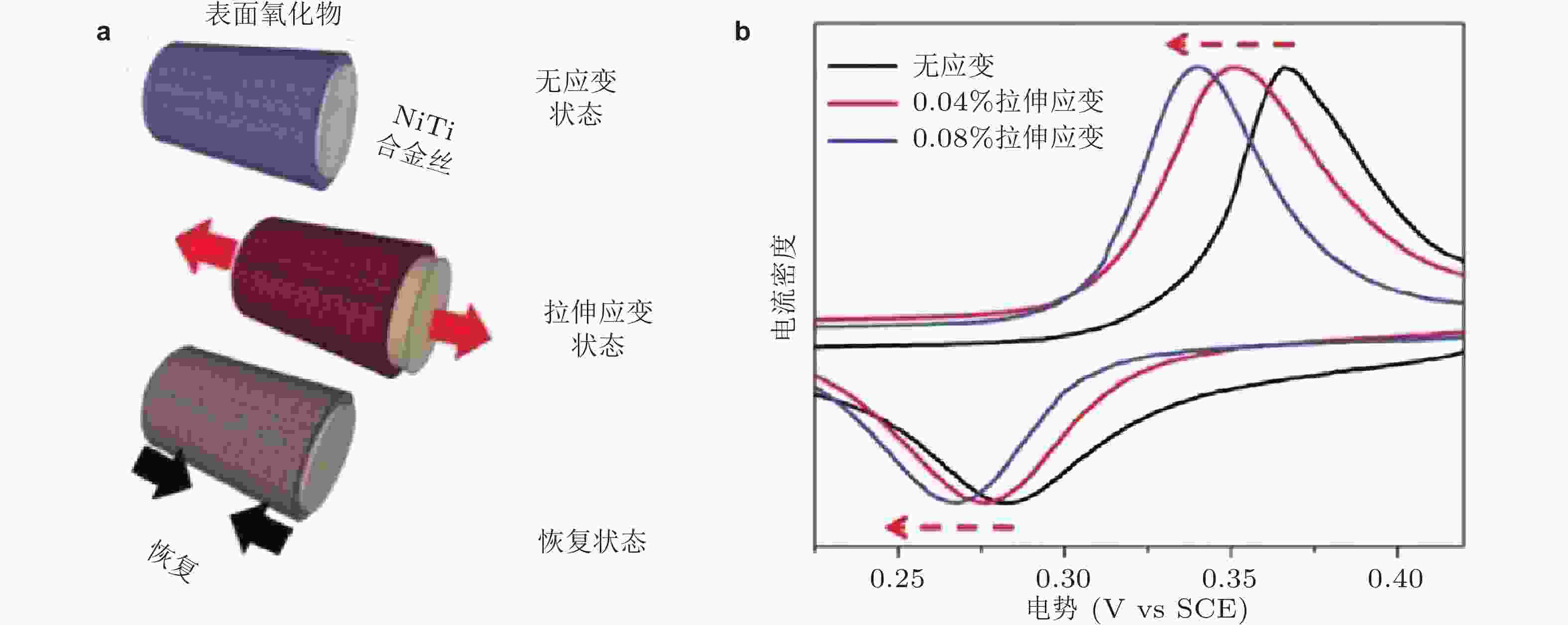
图 10 (a) NiTi合金丝不同应变状态, (b)表面不同应变状态下镍基氧化物薄膜的电化学性能 (Muralidharan et al. 2016)
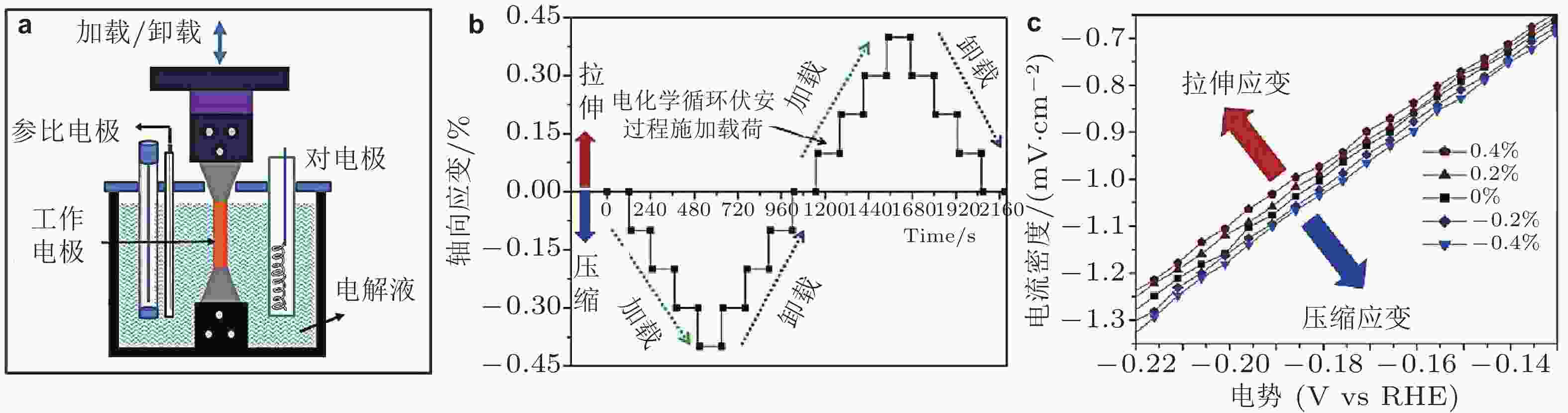
图 11 (a)电化学过程静载荷加载装置示意图, (b)电化学循环伏安过程的加载与卸载, (c)不同拉伸和压缩应变对电化学性能的影响 (Yan et al. 2016)
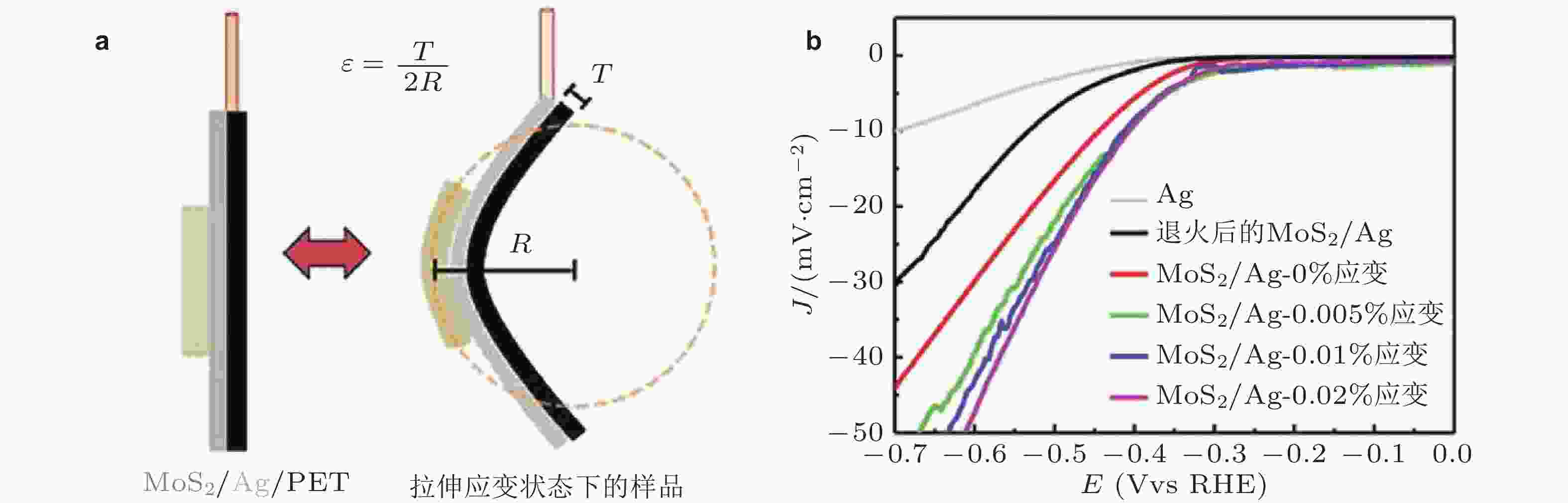
图 12 (a)通过弯曲Ag/PET基底将应变引入到二维材料, 其中R为弯曲半径, T为PET衬底厚度, ε = T/2R为估算的应变大小; (b)与应变相关的析氢反应极化曲线 (Lee et al. 2014)
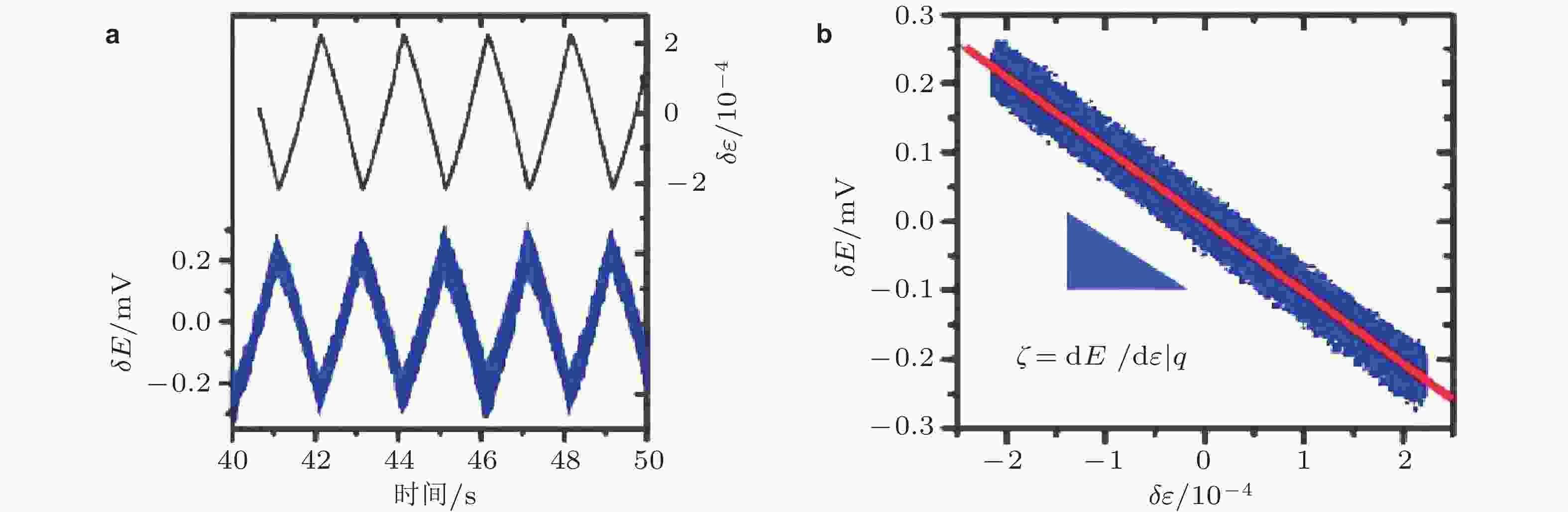
图 13 (a)动态应变以及电极在电解液中的开路电势随时间的变化关系, (b)开路电势与应变呈现出极好的线性负相关性 (Smetanin et al. 2008)
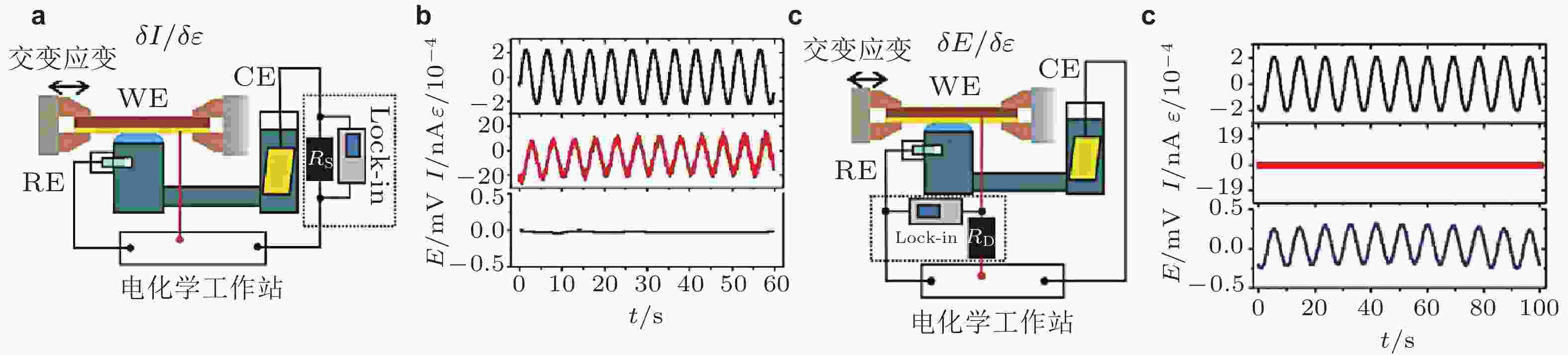
图 14 交变载荷作用下应变效应研究平台电路示意图. (a)电极电流−应变响应; (c) 电极电势−应变响应. WE为工作电极, 即金属薄膜; RE、CE分别为电化学实验中的参比电极和对电极; Lock-In为锁相放大器. (b)和(d)分别为动态机械应变诱导的电流、电势响应信号, 其中横坐标为测量时间, 各个纵坐标分别为应变ε、 电流I、电势E (Smetanin et al. 2011, Deng 2014)
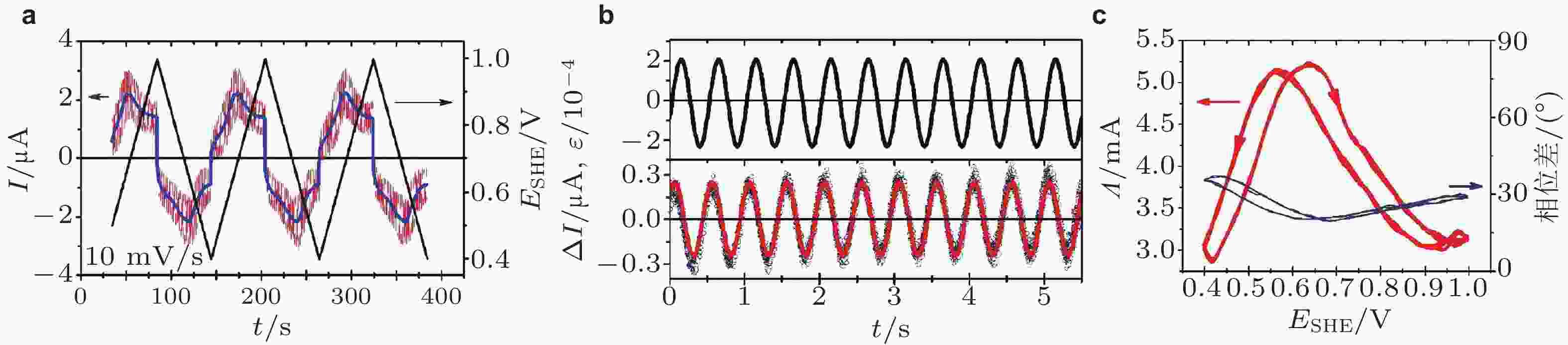
图 15 (a)交变应变作用下循环伏安测试的电流响应信号(I)在时间域(t)的变化, 电极电势扫速为10 mV/s, 施加应变频率υ = 20 Hz, 其中蓝色实线为没有应变作用的常规电流变化情况; (b)恒电势下示波器同步记录的电流变化和频率为2 Hz的应变信号; (c)基于锁定测量技术得到的电流−应变响应系数的幅值和相位结果 (Deng & Yuan 2019)
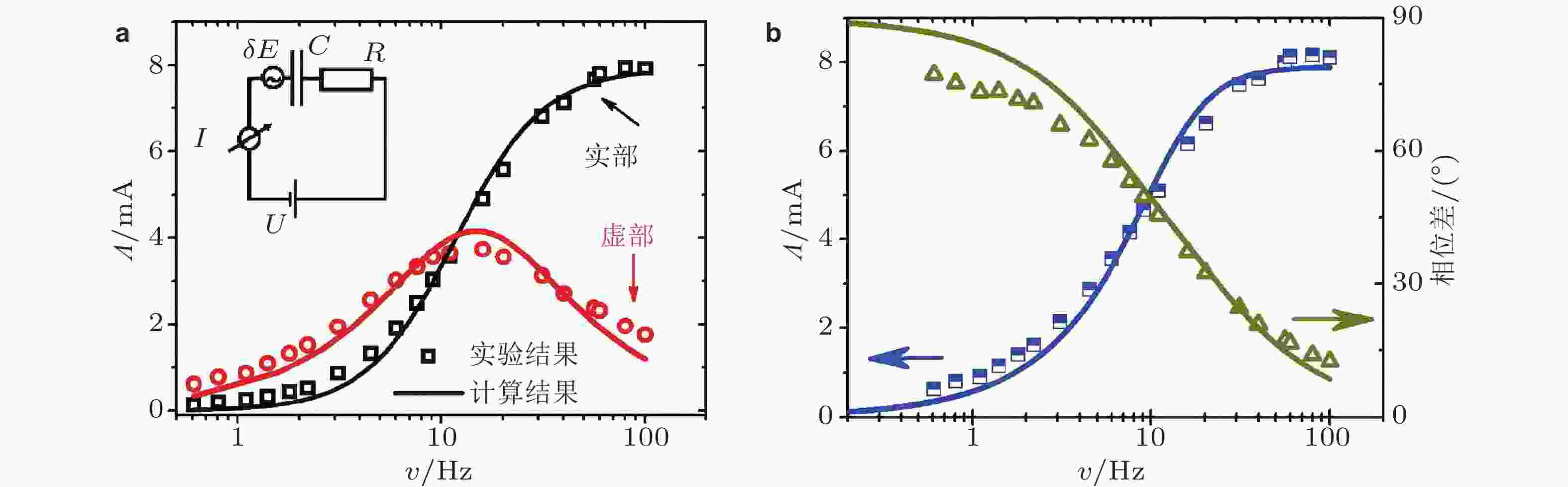
图 16 (a)在电势为0.6 V时, 电流−应变响应系数(Λ)实部、虚部与应变频率υ的函数关系, 插图中金属−电解液界面等效电路模型; (b)电流−应变响应系数(Λ)的幅值和相位移分别与应变频率υ的函数关系. 图中散点为实验数据, 实线为等效电路模拟结果 (Deng & Yuan 2019)
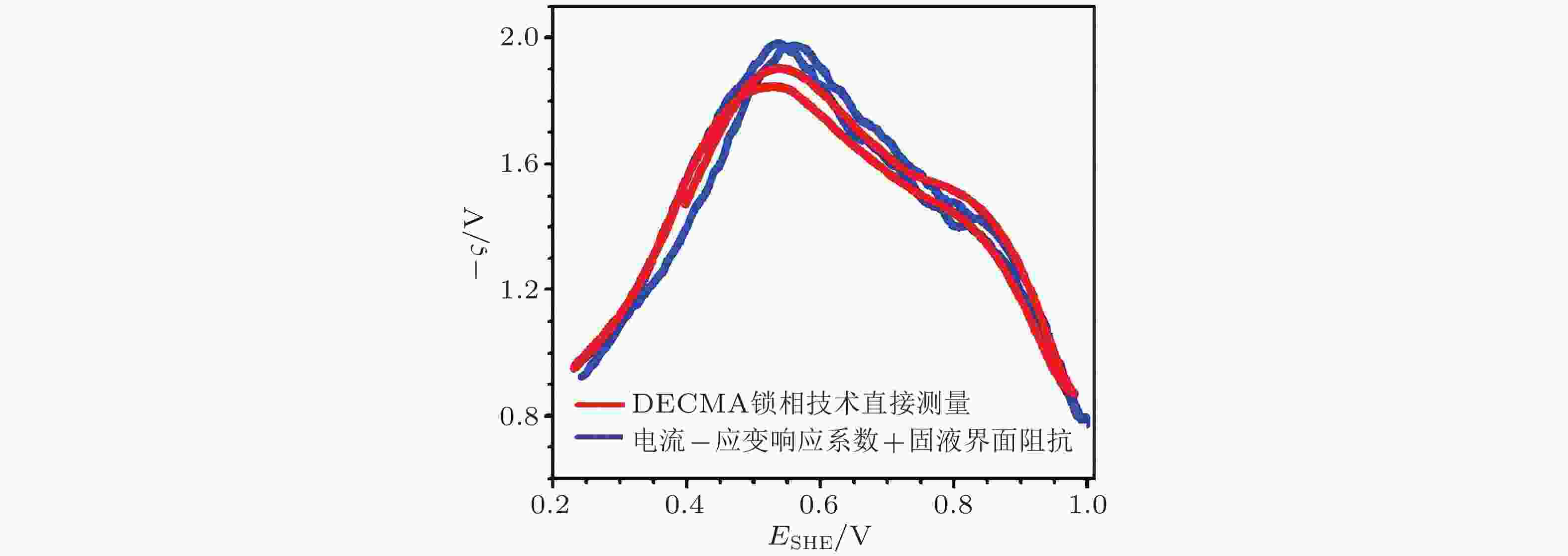
图 17 电势−应变响应系数(ς)的两种测量方法比较: DECMA锁相技术直接测量(红色曲线)以及通过电流−应变响应系数与固液界面阻抗信息(蓝色曲线). 电化学体系: Au薄膜在10 mmol/L的 HClO4溶液中. (Smetanin et al. 2011)
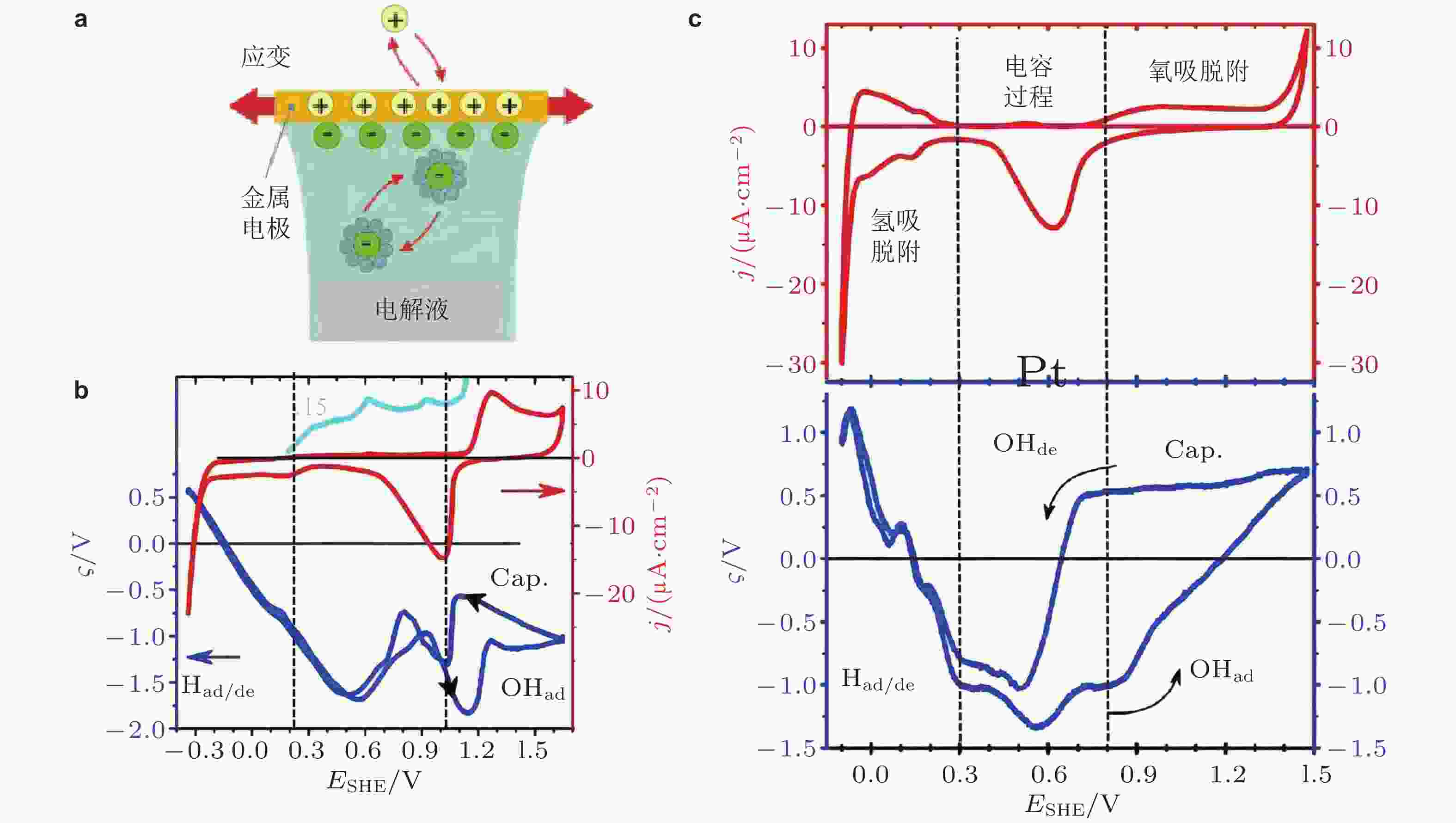
图 18 (a)电化学吸附过程的示意图; 氢离子(H+)和氢氧根(OH−)在金属Au电极表面(b)、Pt电极表面(c)吸附过程的响应系数ς(E). 图中Had/de表示氢吸脱附, OHad/de表示氢氧根吸脱附, Cap.表示电容过程. (Deng & Weissmüller 2014)
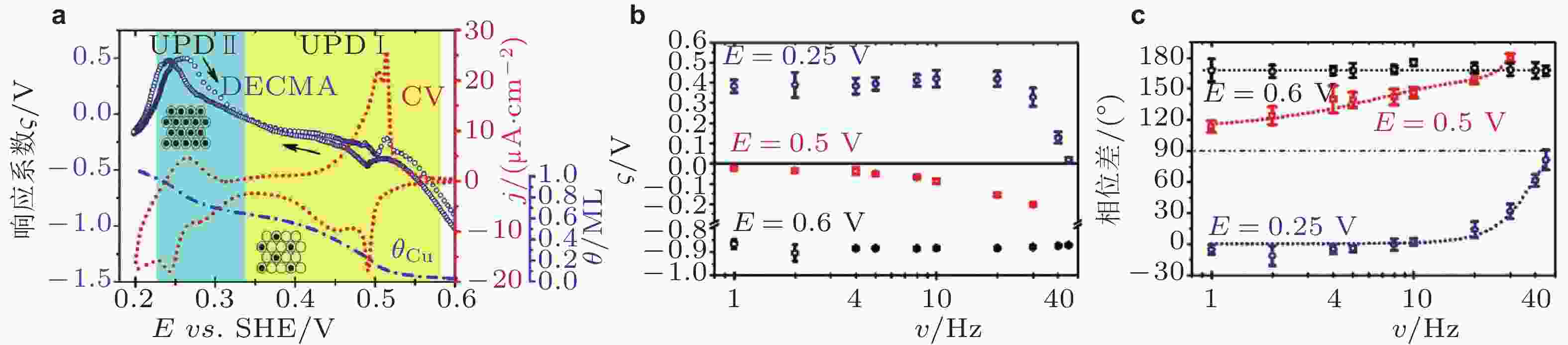
图 19 (a)铜在金电极表面电化学沉积过程响应系数ς与电化学信号、铜原子覆盖度之间的关系; 不同电化学过程响应系数ς的幅值(b)、相位差(c)与应变频率的关系 (Yang et al. 2017)
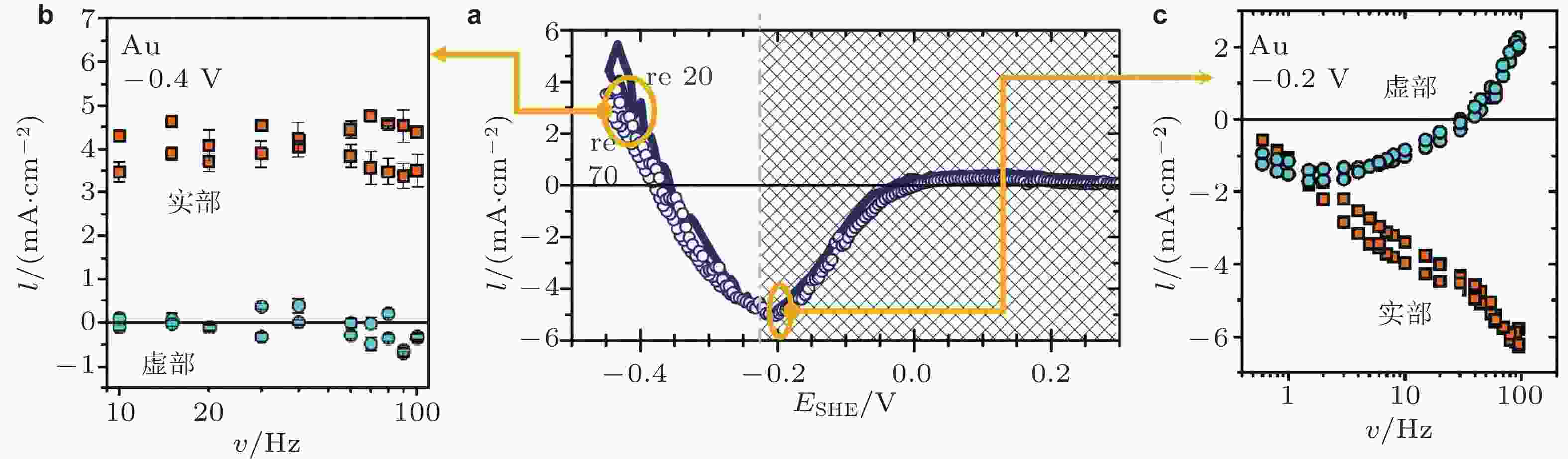
图 20 区分应变对反应电流和双电层电容电流作用的实验结果. (a)反应电流−应变参数 ι 在电化学析氢反应的变化规律, (b)析氢反应占主导时实部和虚部与应变频率的关系, (c)电容过程占主导时实部和虚部与应变频率的关系, 电化学体系为Au在0.5 M H2SO4中 (Deng et al. 2014)
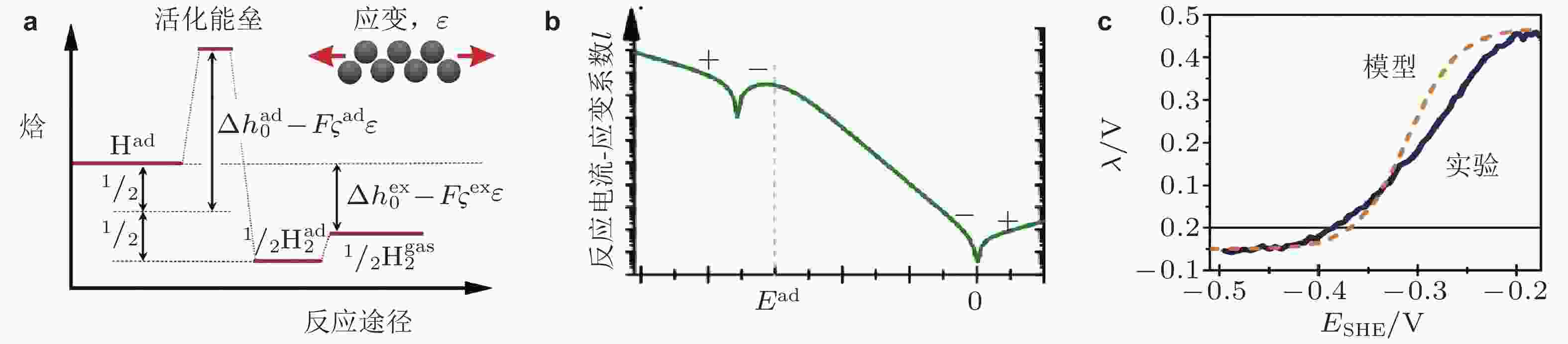
图 21 (a)应变在电化学析氢反应中的作用主要影响吸附焓以及活化焓, (b)数学模型预测的反应电流−应变参数随着电极电势变化规律, (c)表面应变对氢析出电化学反应影响的实验和模型结果对比图 (Deng et al. 2014)
-
[1] 陈贵锋, 刘春峰, 陶俊光. 2018. 表面缺陷促进SnO微板在中性电解质中的电催化性能. 河北工业大学学报, 47: 13-18 (Chen G F, Liu C F, Tao J G. 2018. Surface defects promoted electrocatalytic performance of SnO microplates in neutral electrolyte. Journal of Hebei University of Technology, 47: 13-18 (in Chinese)).Chen GF, Liu CF, Tao JG. 2018. Surface defects promoted electrocatalytic performance of SnO microplates in neutral electrolyte. Journal of Hebei University of Technology, 47(6): 13-18. (in Chinese) [2] 范玮, 秦春玲, 赵维民, 廖波. 2018. 纳米多孔SnSb合金在钠离子电池中的应用. 河北工业大学学报, 47: 37-41 (Fan W, Qin C L, Zhao W M, Liao B. 2018. The nanoporous SnSb alloy electrode for Na-ion batteries application. Journal of Hebei University of Technology, 47: 37-41 (in Chinese)).Fan W, Qin CL, Zhao WM, Liao B. 2018. The nanoporous SnSb alloy electrode for Na-ion batteries application. Journal of Hebei University of Technology, 47(5): 37-41. (in Chinese) [3] 侯健, 杨铮, 贺婷, 李强, 王倩, 张建胜. 2021. 质子交换膜燃料电池热管理问题的研究进展. 中南大学学报(自然科学版), 52: 19-30 (Hou J, Yang Z, He T, Li Q, Wang J, Zhang J S. 2021. Research progress on thermal management of proton exchange membrane fuel cells. Journal of Central South University(Science and Technology) , 52: 19-30 (in Chinese)). doi: 10.11817/j.issn.1672-7207.2021.01.003Hou J, Yang Z, He T, Li Q, Wang J, Zhang JS. 2021. Research progress on thermal management of proton exchange membrane fuel cells. Journal of Central South University(Science and Technology), 52(1): 19−30. (in Chinese) doi: 10.11817/j.issn.1672-7207.2021.01.003 [4] 马增胜, 周益春, 刘军, 薛冬峰, 杨庆生, 潘勇. 2013. 锂离子电池硅负极材料衰退机理的研究进展. 力学进展, 43: 581-599 (Ma Z S, Zhou Y C, Liu J Xue D F, Yang Q S, Pan Y. 2013. Research progress in degradation mechanism of silicon anode materials for lithium-ion batteries. Advances in Mechanics, 43: 581-599 (in Chinese)). doi: 10.6052/1000-0992-13-066Ma ZS, Zhou YC, Liu J Xue DF, Yang QS, Pan Y. 2013. Research progress in degradation mechanism of silicon anode materials for lithium-ion batteries. Advances in Mechanics, 43: 581-599. (in Chinese) doi: 10.6052/1000-0992-13-066 [5] 杨盼盼, 李凯, 冯捷敏, 李大伟, 张俊乾. 2018. 锂离子薄膜电极充放电变形的原位实验观测方法. 实验力学, 33: 716-724 (Yang P P, Li K, Feng J M, Li D W, Zhang J Q. 2018. On the in-situ experimental observation method of lithium-ion thin film electrode deformation in charge-discharge process. Journal of Experimental Mechanics, 33: 716-724 (in Chinese)). doi: 10.7520/1001-4888-18-007Yang PP, Li K, Feng JM, Li DW, Zhang JQ. 2018. On the in-situ experimental observation method of lithium-ion thin film electrode deformation in charge-discharge process. Journal of Experimental Mechanics, 33: 716-724. (in Chinese) doi: 10.7520/1001-4888-18-007 [6] 赵亚溥. 2012. 表面与界面物理力学. 北京, 科学出版社Zhao Y P. 2012. Surface and Interface Mechanics. Beijing: Science Press (in Chinese) [7] 赵亚溥. 2014. 纳米与介观力学. 北京, 科学出版社.Zhao Y P. 2014. Nano and Mesoscopic Mechanics. Beijing: Science Press (in Chinese) [8] 朱博, 孟垂舟, 关玉明, 郭士杰. 2020. 柔性可充电锌空气电池研究进展. 河北工业大学学报, 29: 27-38 (Zhu B, Meng C Z, Guan Y M, Guo S J. 2020. Recent advances in flexible rechargeable zinc-air batteries. Journal of Hebei University of Technology, 29: 27-38 (in Chinese)).Zhu B, Meng CZ, Guan YM, Guo SJ. 2020. Recent advances in flexible rechargeable zinc-air batteries. Journal of Hebei University of Technology, 29(2): 27-38. (in Chinese) [9] 张豪, 林栎阳, 吴博, 胡宁. 2020. 复合材料在柔性电化学储能装置中的应用及研究进展. 河北工业大学学报, 49: 1-20 (Zhang H, Lin L Y, Wu B, Hu N. 2020. Research progress on the application of composite materials in flexible electrochemical energy storage devices. Journal of Hebei University of Technology, 49: 1-20 (in Chinese)).Zhang H, Lin LY, Wu B, Hu N. 2020. Research progress on the application of composite materials in flexible electrochemical energy storage devices. Journal of Hebei University of Technology, 49(3): 1-20. (in Chinese) [10] 张世龙, 冯磊, 李小娜, 王家喜. 2019. 镧改性锌铝氧化物的制备、表征及其催化性能. 河北工业大学学报, 48: 29-34 (Zhang S L, Feng L, Li X A, Wang J X. 2019. Preparation and characterization of lanthanum modified zinc aluminum oxide and its catalytic performance. Journal of Hebei University of Technology, 48: 29-34 (in Chinese)).Zhang SL, Feng L, Li XA, Wang JX. 2019. Preparation and characterization of lanthanum modified zinc aluminum oxide and its catalytic performance. Journal of Hebei University of Technology, 48(1): 29-34. (in Chinese) [11] Bu L Z, Zhang N, Guo S J, Zhang X, Li J, Yao J L, Wu T, Lu G, Ma J Y, Su D, Huang X Q. 2016. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science, 354: 1410-1414. doi: 10.1126/science.aah6133 [12] Burebi Y, Jia Z, Qu S X. 2019. A chemo-mechanical model for fully-coupled lithiation reaction and stress generation in viscoplastic lithiated silicon. Science China: Technological Sciences, 62: 1365-1374. doi: 10.1007/s11431-018-9499-x [13] Cahn JW. 1978. Thermodynamics of solid and fluid surfaces. ASM, Metals Park, Ohio: 3-23. [14] Cheng W R, Zhao X, Su H, Tang F M, Che W, Zhang H, Liu Q H. 2019. Lattice-strained metal-organic-framework arrays for bifunctional oxygen electrocatalysis. Nature Energy, 4: 115-122. doi: 10.1038/s41560-018-0308-8 [15] Deng Q B. 2014. Dynamic electro-chemo-mechanical analysis at the metal-electrolyte interface. Technische Universität Hamburg, Germany. [16] Deng Q B, Smetanin M, Weissmüller J. 2014. Mechanical modulation of reaction rate in electrocatalysis. Journal of Catalysis, 309: 351-361. [17] Deng Q B, Weissmüller J, 2014. Electrocapillary coupling during electrosorption. Langmuir, 30: 10522-10530. [18] Deng Q B, Gopal V, Weissmüller J. 2015a. Less noble or more noble: How strain affects the binding of oxygen on gold. Angewandte Chemie International Edition, 54: 12981-12985. doi: 10.1002/anie.201504715 [19] Deng Q B, Gosslar D H, Smetanin M, Weissmüller J. 2015b. Electrocapillary coupling at rough surface. Physical Chemistry Chemical Physics, 17: 11725-11731. doi: 10.1039/C5CP00167F [20] Deng Q B, Yuan A T. 2019. Monitoring and modeling the variation of electrochemical current induced by dynamic strain at gold surfaces. Journal of The Electrochemical Society, 166: H480-H484. doi: 10.1149/2.0081912jes [21] Du M S, Cui L S, Cao Y, Bard A J. 2015. Mechanoelectrochemical catalysis of the effect of elastic strain on a platinum nanofilm for the ORR exerted by a shape memory alloy substrate. Journal of the American Chemical Society, 137: 7397-7403. doi: 10.1021/jacs.5b03034 [22] Du M S, Wan Q, Wang Z Q, Cui L S. 2016. Elastic strain induced improvement in the photocatalytic activity of semiconducting film exerted by the surface relief of Fe-Ni-Co-Ti alloy substrate. Materials Letters, 168: 192-195. doi: 10.1016/j.matlet.2016.01.057 [23] Eftekhari A. 2017. Tuning the electrocatalysts for oxygen evolution reaction. Materials Today Energy, 5: 37-57. doi: 10.1016/j.mtener.2017.05.002 [24] Gauthier Y, Schmid M, Padovani S, Lundgren E, Bus V, Kresse G, Redinger J, Varga P. 2001. Adsorption sites and ligand effect for CO on an alloy surface: A direct view. Physical Review Letters, 87: 036103. doi: 10.1103/PhysRevLett.87.036103 [25] Gennero de Chialvo M R, Chialvo A C. 1994. Hydrogen evolution reaction: analysis of the Volmer-Heyrovsky-Tafel mechanism with a generalized adsorption model. Journal of Electroanalytical Chemistry, 372: 209-223. doi: 10.1016/0022-0728(93)03043-O [26] Guo S, Zhang X, Zhu W, He K, Su D, Mendoza-Garcia A, Ho S F, Lu G, Sun S. 2014. Nanocatalyst superior to Pt for oxygen reduction reactions: The case of core/shell Ag(Au)/CuPd nanoparticles. Journal of the American Chemical Society, 136: 15026-15033. doi: 10.1021/ja508256g [27] Gsell M, Jakob P, Menzel D. 1998. Effect of substrate strain on adsorption. Science, 280: 717-720. doi: 10.1126/science.280.5364.717 [28] Gurtin M E, Murdoch A I. 1975. A continuum theory of elastic material surfaces. Arch Ration Mech Anal, 57: 291-323. doi: 10.1007/BF00261375 [29] Hammer B, Nørskov J K. 1995. Electronic factors determining the reactivity of metal surfaces. Surf. Sci, 343: 211-220. [30] Han L, Dong S J, Wang E K. 2016. Transition-metal (Co, Ni, and Fe)-based electrocatalysts for the water oxidation reaction. Advanced Materials, 26: 9266-9291. [31] He J, Shen Y L, Yang M Z, Zhang H X, Deng Q B, Ding Y. 2017. The effect of surface strain on the CO-poisoned surface of Pt electrode for hydrogen adsorption. Journal of Catalysis, 350: 212-217. doi: 10.1016/j.jcat.2017.04.004 [32] Huang H W, Jia HH, Liu Z, Gao P F, Zhao J T, Luo Z L, Yang J L, Zeng J. 2017. Understanding of strain effect in electrochemical reduction of CO2 : Using Pd nanostructures as an ideal platform. Angewandte Chemie International Edition 56(13): 3594-3598 [33] Khorshidi A, Violet J, Hashemi J, Peterson A A. 2018. How strain can break the scaling relations of catalysis. Nature Catalysis, 1: 263-268. doi: 10.1038/s41929-018-0054-0 [34] Kitchin J R, Norskov J K, Barteau M A, Chen J G. 2004. Role of strain and ligand effects in the modification of the electronic and chemical properties of bimetallic surfaces. Physical Review Letters, 95: 156801. [35] Kibler L A, El-Aziz A M, Hoyer R, Kolb D M. 2005. Tuning reaction rates by lateral strain in a palladium monolayer. Angewandte Chemie-International Edition, 44: 2080-2084. doi: 10.1002/anie.200462127 [36] Kibler L A. 2006. Hydrogen electrocatalysis. Chem Phys Chem., 7: 985-991. doi: 10.1002/cphc.200500646 [37] Kibler L A. 2008. Dependence of electrocatalytic activity on film thickness for the hydrogen evolution reaction of Pd overlayers on Au(111). Electrochimica Acta, 53: 6824-6828. doi: 10.1016/j.electacta.2008.01.097 [38] Kramer D, Weissmüller J. 2007. A note on surface stress and surface tension and their interrelation via Shuttleworth's equation and the Lippmann equation. Surface Science, 601: 3042-3051. doi: 10.1016/j.susc.2007.05.005 [39] Lafouresse M C, Bertocci U, Beauchamp C R, Stafford G R. 2012. Simultaneous electrochemical and mechanical impedance spectroscopy using cantilever curvature. Journal of the Electrochemical Society, 159: H816-H822. doi: 10.1149/2.055210jes [40] Lee J H, Jang W S, Han S W, Baik H K. 2014. Efficient hydrogen evolution by mechanically strained MoS2 nanosheets. Langmuir, 230: 9866-9873. [41] Li H, Tsai C, Koh A L, et al. 2016. Activating and optimizing MoS2 basal planes for hydrogen evolution through the formation of strained sulphur vacancies. Nature Materials, 15: 48-53. doi: 10.1038/nmat4465 [42] Li Y, Zhang J, Zhang K, Zheng B L, Yang F Q. 2019. A defect-based viscoplastic model for large-deformed thin film electrode of lithium-ion battery. International Journal of Plasticity, 115: 293-306. doi: 10.1016/j.ijplas.2018.12.001 [43] Li Y R, Li M X, Li S N. et al. 2021. A review of energy and environment electrocatalysis based on high-index faceted nanocrystals. Rare Metals, 40: 3406-3441. doi: 10.1007/s12598-021-01747-8 [44] Liang H Z, Zhang X Y, Yang L, Wu Y K, Fang D N. 2019. Electro-chemomechanical coupled behaviors of deformation and failure in electrode materials for lithium-ion batteries. Science China: Technological Sciences, 62: 1277-1296. doi: 10.1007/s11431-018-9485-6 [45] Liu B H, Wang X, Chen H S, Chen S, Yang H X, Xu J, Jiang H Q, Fang D N. 2019. A simultaneous multiscale and multiphysics model and numerical implementation of a core-shell model for lithium-ion full-cell batteries. Journal of Applied Mechanics, 86: 041005. doi: 10.1115/1.4042432 [46] Luo M C, Guo S J. 2017. Strain-controlled electrocatalysis on multimetallic nanomaterials. Nature Reviews Materials, 2: 17059. doi: 10.1038/natrevmats.2017.59 [47] Ma Z, Wu H, Wang Y, Pan Y, Lu C. 2017. An electrochemical-irradiated plasticity model for metallic electrodes in lithium-ion batteries. International Journal of Plasticity, 88: 188. [48] Maark T A, Peterson A A. 2014. Understanding strain and ligand effects in hydrogen evolution over Pd(111) surfaces. Journal of Physical Chemistry C, 118: 4275-4281. doi: 10.1021/jp4121035 [49] Masa J, Andronescu C, Antoni H, Sinev I, Seisel S, Elumeeva K, Barwe S, Marti-Sanchez S, Arbiol J, Roldan Cuenya B, et al. 2019. Role of boron and phosphorus in enhanced electrocatalytic oxygen evolution by nickel borides and nickel phosphides. ChemElectroChem, 6: 235-240. doi: 10.1002/celc.201800669 [50] Mavrikakis M, Hammer B, Nørskov J K. 1998. Effect of strain on the reactivity of metal surfaces. Physical Review Letters, 81: 2819-2822. doi: 10.1103/PhysRevLett.81.2819 [51] Mavrikakis M, Stoltze P, Nørskov J K. 2000. Making gold less noble. Catalysis letters, 64: 101-106. [52] Muralidharan N, Carter R, Oakes L, Coakes A P, Oint C L. 2016. Strain engineering to modify the electrochemistry of energy storage electrodes. Scientific Reports, 6: 27542. doi: 10.1038/srep27542 [53] Murdoch A I. 1976. A thermodynamical theory of elastic material interfaces. The Quarterly Journal of Mechanics and Applied Mathematics, 29: 245-275. doi: 10.1093/qjmam/29.3.245 [54] Niu Z, Wan Y, Li X, Zhang M, Lu G, Yan K. 2020. In-situ regulation of formic acid oxidation via elastic strains. Journal of Catalysis, 389: 631-635. doi: 10.1016/j.jcat.2020.07.004 [55] Parsons R. 1958 The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen. Trans. Faraday Soc. , 54: 1053-1063. [56] Schlapka A, Lischka M, Groß A, Kӓsberger U, Jakob P. 2003. Surface strain versus substrate interaction in heteroepitaxial metal layers: Pt on Ru (0001). Physical Review Letters, 91: 016101. doi: 10.1103/PhysRevLett.91.016101 [57] Schnur S, Groß A. 2010. Strain and coordination effects in the adsorption properties of early transition metals: A density-functional theory study. Physical Review B, 81: 033402. [58] Seh Z W, Kibsgaard J, Dickens C F, Chorkendorff I B, Norskov J K, Jaramillo T F. 2017. Combining theory and experiment in electrocatalysis: Insights into materials design. Science, 355: eaad4998. doi: 10.1126/science.aad4998 [59] Sheng W, Gasteiger H A, Shao-Horn Y. 2010. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. Journal of The Electrochemical Society, 157: B1529-B1536. doi: 10.1149/1.3483106 [60] Smetanin M, Deng Q B, Weissmüller J. 2011. Dynamic electro-chemo-mechanical analysis during cyclic voltammetry. Physical Chemistry Chemical Physics, 13: 17313-17322. doi: 10.1039/c1cp21781j [61] Smetanin M, Kramer D, Mohanan S, Herr U, Weissmüller J. 2009. Response of the potential of a gold electrode to elastic strain. Physical Chemistry Chemical Physics, 11: 9008-9012. doi: 10.1039/b913448d [62] Smetanin M, Viswanath R N, Kramer D, Beckmann D, Koch T, Kibler L A, Kolb D M, Weissmüller J. 2008. Surface stress-charge response of a (111)-textured gold electrode under conditions of weak ion adsorption. Langmuir, 24: 8561-8567. doi: 10.1021/la704067z [63] Strasser P, Koh S, Anniyev T, Greeley J, More K, Yu C F, Liu Z C, Kaya S, Nordlund D, Ogasawara H, et al. 2010. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nature Chemistry, 2: 454-460. doi: 10.1038/nchem.623 [64] Tavassol H, Jones E M C, Sottos N R, Gewirth A A. 2016. Electrochemical stiffness in lithium-ion batteries. Nature Materials, 15: 1182. doi: 10.1038/nmat4708 [65] Temmel S E, Fabbri E, Pergolesi D, Lippert T, Schmidt T J. 2016. Investigating the role of strain toward the oxygen reduction activity on model thin film Pt catalysts. ACS Catalysis, 6: 7566-7576. doi: 10.1021/acscatal.6b01836 [66] Wang A Q, Zhao Z L, Hu D, Niu J F, Zhang M, Yan K, Lu G. 2019. Tuning the oxygen evolution reaction on a nickel–iron alloy via active straining. Nanoscale, 11: 426-430. doi: 10.1039/C8NR08879A [67] Wang H, Shan X, Yu H, Wang Y, Schmickler W, Chen H Y, Tao N. 2017. Determining electrochemical surface stress of single nanowires. Angewandte Chemie International Edition, 129: 2164-2135. [68] Wang H T, Xu S C, Tsai C, Li Y Z, Liu C, Zhao J, Liu Y Y, Yuan H Y, Abild-Pedersen F, Prinz F B, et al. 2016. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science, 354: 1031-1036. doi: 10.1126/science.aaf7680 [69] Wang L, Stoerzinger K A, Chang L, Yin X M, Li Y Y, Tang C S, Jia E D, Bowden M E, Yang Z Z, Abdelsamie A, et al. 2019b. Strain effect on oxygen evolution reaction activity of epitaxial NdNiO3 thin films. ACS Applied Materials & Interfaces, 11: 12941-12947. [70] Wang L, Zeng Z H, Gao W P, Maxson T, Raciti D, Giroux M, Pan X Q, Wang C, Greeley J, Wang J. 2019a. Tunable intrinsic strain in two-dimensional transition metal electrocatalysts. Science, 363: 870. doi: 10.1126/science.aat8051 [71] Wang P, Qu W J, Song W L, Chen H S, Chen R J, Fang D N. 2019. Electro-chemo-mechanical issues at the interfaces in solid-state lithium metal batteries. Advanced Functional Materials, 29: 1900950. [72] Wang X, Choi S I, Roling L T, et al. 2015. Palladium-platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nature Communication, 6: 7594. doi: 10.1038/ncomms8594 [73] Wang Z Q, Zhao Y P. 2011. Thermo-hyperelastic models for nanostructured materials. Science China-Physics Mechanics & Astronomy, 54: 948-956. [74] Weissmüller J, Kramer D. 2005. Balance of force at curved solid metal–liquid electrolyte interfaces. Langmuir, 21: 4592-4603. doi: 10.1021/la047838a [75] Weissmüller J. 2013. “Electrocapillarity of solids and its impact on heterogeneous catalysis” in Electrocatalysis: Theoretical Foundations and Model Experiments. Electrocatalysis, 163-220. [76] Xie H M, Zhang Q, Song H B, Shi B Q, Kang Y L. 2017. Modeling and in situ characterization of lithiation-induced stress in electrodes during the coupled mechano-electro-chemical process. Journal of Power Sources, 342: 896-903. doi: 10.1016/j.jpowsour.2017.01.017 [77] Xu R, Zhao K J. 2018. Corrosive fracture of electrodes in Li-ion batteries. Journal of the Mechanics and Physics of Solids, 121: 258-280. doi: 10.1016/j.jmps.2018.07.021 [78] Xue Y Y, Ge H, Chen Z, Zhai, Y B, Zhang J, Sun J Q, Abbas M, Lin K, Zhao W T, Chen J G. 2018. Effect of strain on the performance of iron-based catalyst in Fischer-Tropsch synthesis. Journal of Catalysis, 358: 237-242. doi: 10.1016/j.jcat.2017.12.017 [79] Yan K, Maark T A, Khorshidi A, et al. 2016. The influence of elastic strain on catalytic activity in the hydrogen evolution reaction. Angewandte Chemie-International Edition, 55: 6175-6181. doi: 10.1002/anie.201508613 [80] Yan K, Kim S K, Khorshidi A, et al. 2017. High elastic strain directly tunes the hydrogen evolution reaction on tungsten carbide. The Journal of Physical Chemistry C, 121: 6177-6183. doi: 10.1021/acs.jpcc.7b00281 [81] Yang M Z, Zhang H X, Deng Q B. 2017. Understanding the copper underpotential deposition process at strained gold surface. Electrochemistry Communications, 82: 125-128. [82] Yao J D, Huang W J, Fang W, Kuang M, Jia N, Ren H, Liu D B, Lv C D, Liu C T, Xu J W, et al. 2020. Promoting electrocatalytic hydrogen evolution reaction and oxygen evolution reaction by fields: Effects of electric field, magnetic field strain and light. Small Methods, 4: 2000494. doi: 10.1002/smtd.202000494 [83] You B, Tang M T, Tsai C, Abild-Pedersen F, Zheng X L, Li H. 2019. Enhancing electrocatalytic water splitting by strain engineering. Advanced Materials, 31: 1807001. doi: 10.1002/adma.201807001 [84] Yuan A T, Zhang H X, Deng D B. 2019. A simple mechanical method to modulate the electrochemical electrosorption processes at metal surfaces. Molecules 24: 3662. [85] Zhang H X, An C H, Yuan A T, Deng Q B, Ning J L. 2018. A non-conventional way to modulate the capacitive process on carbon cloth by mechanical stretching. Electrochemistry Communications, 89: 43. doi: 10.1016/j.elecom.2018.02.017 [86] Zhao K J, Cui Y. 2016. Understanding the role of mechanics in energy materials: A perspective. Extreme Mechanics Letters 9: 347-352. [87] Zhu T, Fang X F, Wang B L. 2019. Challenges and opportunities in chemomechanics of materials: A perspective. Science China: Technological Sciences, 62: 1385-1387. doi: 10.1007/s11431-018-9516-2 -







 下载:
下载: