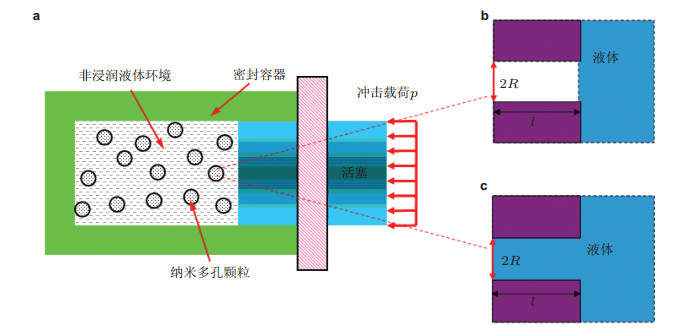
-
摘要: 基于纳米流控行为设计的新一代能量吸收耗散系统(nanofluidic en-ergy absorption system,NEAS)将会比传统吸能材料具有更高的能量吸收密度,而且还可以重复使用,特别是在小体积应用环境下具有显著的优势.本文从实验和计算模拟两方面综述了目前关于NEAS能量吸收耗散行为的最新研究进展,其中实验研究主要包括准静态压缩和动态压缩测试,计算模拟研究主要是采用基于经验势的分子动力学模拟方法.通过准静态压缩实验,可以测量NEAS模型的载荷-位移关系曲线,从而获得NEAS模型的临界渗透压强,了解卸载后系统是否能够恢复到加载前的状态(即是否可以重复使用),并通过载荷-位移关系曲线下面积估算NEAS模型的吸能密度;通过动态压缩实验可以测量NEAS模型对脉冲载荷的缓冲保护作用,主要体现为降低脉冲载荷幅值和扩展脉冲宽度.计算模型研究可以明确给出NEAS对外载荷的微观响应,从而可以准确了解NEAS的能量吸收耗散机制以及吸能密度的主要影响因素.本研究可以帮助我们全面了解NEAS的研究进展,为NEAS的设计与优化提供重要指导.Abstract: The energy absorption system designed on the basis of nanofluidic behavior (also called nanofluidic energy absorption system, NEAS) will have a higher energy ab-sorption density than the conventional energy absorption materials, and can be repeatedly used. Thus it shows great advantages over the conventional energy absorption materials, especially for applications with a limited volume. In this paper, we reviewed the state-of-the-art of the energy absorption behavior of NEAS from both experimental investigations and numerical studies:the experimental work mainly includes quasi-static compression and dynamic compression tests; the computational simulations are mainly based on molecular dynamics simulations developed from the empirical potentials. Using quasi-static compres-sion, we can measure the load-displacement relationship of NEAS, determine the critical infiltration pressure, understand the loading-unloading-reloading behavior of NEAS (closely related to the repeated energy absorption performance of NEAS), and estimate the energy absorption density from the area below the load-displacement curve. By use of the dynamic compression tests, the NEAS performance of the protection against the impact load can be measured, which can be represented by decreasing the impact pulse magnitude and expand-ing the pulse width. The computational studies can clearly show the micro-level response of NEAS to the external load, based on which we can fully understand the energy absorption mechanism and the main controlling parameters of energy absorption density. The present study can help researchers understand the latest research progress of NEAS, and provide an important guideline for the design and optimization of NEAS.
-
图 3 NEAS模型准静态压缩下的加卸载响应. (a)沸石系统+水构成的悬浊液(最早的NEAS模型) (Eroshenko et al. 2001), (b) NEAS模型(纳米多孔硅胶颗粒MTS+水溶液) (Lefevre et al. 2004), (c) NEAS模型(纳米多孔硅胶颗粒Fluke-C8+水溶液)的两次加卸载循环(Qiao et al. 2007), (d) NEAS模型(纳米多孔硅胶颗粒Fluke-C8+水溶液)在加载后加压保持12 h, 然后卸载, 再进行第2次加卸载循环(Qiao et al. 2007)
图 4 (a)注入NEAS (纳米多孔硅胶颗粒+水溶液)的不锈钢管准静态压缩实验图, (b)注入NEAS (纳米多孔硅胶颗粒+水溶液)的不锈钢管(黑色粗实线)的准静态加载响应(Chen et al. 2006), 注入纯水的不锈钢管(红色细实线)和纯不锈钢管(虚线)的准静态加载响应
图 5 NEAS模型(水+沸石(Zeolite β))和传统吸能材料泡沫铝的吸能密度比较(Sun et al. 2015)
图 6 (a) NEAS模型(水+沸石(Zeolite β))的吸能缓冲作用, (b) NEAS模型(水+沸石(Zeolite β))动态响应和准静态响应比较(Sun et al. 2015)
图 7 NEAS的MD计算模型. (a)无底部纳米通道模型(Cao 2012), (b)有底部纳米通道模型(Liu & Cao 2013b), (c)锥形纳米通道模型(Liu et al. 2009c), (d)变截面纳米通道模型(Hu et al. 2016), (e)冲击质量块作用模型(Xu et al. 2014), (f)落锤直接作用水池模型(Liu & Cao 2016b)
图 8 MD模拟中落锤冲击加载模式产生的压强脉冲波(Liu & Cao 2016b)
图 9 纳米尺度下Young{Laplace方程有效性检验结果(Liu & Cao 2016b). (a)水池压强与孔上液面曲率半径关系, (b)纳米尺度下温度和电解质(NaCl)对水的表面曲率半径和液体压强之间的关系的影响
图 11 NEAS模型纳米通道界面能密度随渗透水分子数的变化(Liu & Cao 2013b). (a) R=0:67 nm, (b) R=1:0 nm, (c) R=1:33 nm
图 12 NEAS模型纳米通道界面能密度随管径的变化(Liu & Cao 2013b)
图 13 NEAS模型在落锤冲击作用下的完整加卸载响应(R=1:0 nm). (a) CNT内的渗入水分子数随时间的变化, (b)界面能随时间的变化(Liu & Cao 2016b)
图 14 落锤冲击速度、质量和冲击能对NEAS模型冲击响应的影响. (a)在落锤冲击作用下纳米通道充满时的渗透水分子数, (b)所形成的界面能(Liu & Cao 2016b)
图 15 具有粗糙管壁的NEAS模型对落锤冲击的响应(A/R0=0:1, 80 m/s). (a)在落锤冲击作用下粗糙管壁纳米通道内渗透水分子数随时间的变化, (b)所形成的界面能随时间的变化(Liu & Cao 2016b)
图 16 NEAS吸能密度随管径的变化. (a)根据单根CNT体积估算, (b)根据整体NEAS模型的体积(CNT阵列+水)估算(图中数据点上的误差范围对应于CNT阵列所占NEAS的体积分数(0.33~0.50) (Liu & Cao 2013b)
图 17 NEAS的吸能密度随甘油溶液浓度的变化, 图中数据点上误差范围反映的是冲击速度对吸能密度的影响(v=100~1 000 m/s), 吸能密度随冲击速度的增加而增大(Liu & Cao 2014)
表 1 不同吸能耗散系统的吸能密度

-
[1] 卢子兴. 2002.微孔泡沫塑料力学行为的研究综述.力学进展, 32:365-378. http://lxjz.cstam.org.cn/CN/abstract/abstract132203.shtmlLu Z X. 2002. A review of studies on the mechanical properties of microcellular plastics. Advances in Mechanics, 32:365-378. http://lxjz.cstam.org.cn/CN/abstract/abstract132203.shtml [2] 杨亚政, 杨嘉陵, 曾涛, 方岱宁. 2007.轻质多孔材料研究进展.力学季刊, 28:503-514. http://www.cnki.com.cn/Article/CJFDTOTAL-SHLX200704002.htmYang Y Z, Yang J L, Zheng T, Fang D N. 2007. Progress in research work of light materials. Chinese Quarterly of Mechanics, 28:503-514 http://www.cnki.com.cn/Article/CJFDTOTAL-SHLX200704002.htm [3] 余同希, 卢国兴. 2006.材料与结构的能量吸收-耐撞性.包装.安全防护.北京:化学工业出版社.Yu T X, Lu G X. 2006. Energy Absorption of Structures and Materials. Beijing:Chemical Industry Press [4] Abascal J L F, Sanz E, Vega C. 2009. Triple points and coexistence properties of the dense phases of water calculated using computer simulation. Physical Chemistry Chemical Physics, 11:556-562. doi: 10.1039/B812832D [5] Alghamdi A A A. 2001. Collapsible impact energy absorbers:An overview. Thin-Walled Structures, 39:189-213. doi: 10.1016/S0263-8231(00)00048-3 [6] Arash B, Wang Q, Varadan V K. 2011. Carbon nanotube-based sensors for detection of gas atoms. Journal of Nanotechnology in Engineering and Medicine, 2:021010. doi: 10.1115/1.4003967 [7] Arash B, Wang Q, Wu N. 2012. Gene detection with carbon nanotubes. Journal of Nanotechnology in Engineering and Medicine, 3:020902. doi: 10.1115/1.4007388 [8] Bhatt D, Newman J, Radke C J. 2004. Molecular dynamics simulations of surface tensions of aqueous electrolytic solutions. Journal of Physical Chemistry B, 108:9077-9084. doi: 10.1021/jp037212d [9] Cao G X. 2012. Working mechanism of nanoporous energy absorption system under high speed loading.Journal of Physical Chemistry C, 116:8278-8286. doi: 10.1021/jp3009145 [10] Cao G X, Qiao Y, Zhou Q L, Chen X. 2008a. Infiltration behaviour of water in a carbon nanotube under external pressure. Philosophical Magazine Letters, 88:371-378. doi: 10.1080/09500830802050415 [11] Cao G X, Qiao Y, Zhou Q L, Chen X. 2008b. Water infiltration behaviours in carbon nanotubes under quasi-static and dynamic loading conditions. Molecular Simulation, 34:1267-1274. doi: 10.1080/08927020802175225 [12] Chen F, Smith P E. 2007. Simulated surface tensions of common water models. Journal of Chemical Physics, 126:221101. doi: 10.1063/1.2745718 [13] Chen X, Cao G X, Han A J, Punyamurtula V K, Liu L, Culligan P J, Kim T, Qiao Y. 2008. Nanoscale fluid transport:Size and rate effects. Nano Letters, 8:2988-2992. doi: 10.1021/nl802046b [14] Chen X, Surani F B, Kong X G, Punyamurtula V K, Qiao Y. 2006. Energy absorption performance of steel tubes enhanced by a nanoporous material functionalized liquid. Applied Physics Letters, 89:241918. doi: 10.1063/1.2405852 [15] Chen X, Xu B X, Liu L. 2014. Nanoscale fluid mechanics and energy conversion. Applied Mechanics Reviews, 66:050803. doi: 10.1115/1.4026913 [16] Chitra R, Smith P E. 2001. A comparison of the properties of 2, 2, 2-trifluoroethanol and 2, 2, 2-trifluoroethanol/water mixtures using different force fields. Journal of Chemical Physics, 115:5521-5530. doi: 10.1063/1.1396676 [17] Cohen-Tanugi D, Grossman J C. 2012. Water desalination across nanoporous graphene. Nano Letters, 12:3602-3608. doi: 10.1021/nl3012853 [18] Cottin-Bizonne C, Barentin C, Charlaix E, Bocquet L, Barrat J L. 2004. Dynamics of simple liquids at heterogeneous surfaces:Molecular-dynamics simulations and hydrodynamic description. European Physical Journal E, 15:427-438. doi: 10.1140/epje/i2004-10061-9 [19] Eijkel J. 2007. Liquid slip in micro-and nano-fluidics:Recent research and its possible implications. Lab on a Chip, 7:299-301. doi: 10.1039/b700364c [20] Eroshenko V A, Fadeev A Y. 1995. Intrusion and extrusion of water in hydrophobized porous silica. Colloid Journal, 57:446-449. https://www.researchgate.net/publication/279667080_Intrusion_and_extrusion_of_water_in_hydrophobized_porous_silica [21] Eroshenko V, Regis R C, Soulard M, Patarin J. 2001. Energetics:A new field of applications for hydrophobic zeolites. Journal of the American Chemical Society, 123:8129-8130. doi: 10.1021/ja011011a [22] Evans A G, Hutchinson J W, Fleck N A, Ashby M F, Wadley H N G. 2001. The topological design of multifunctional cellular metals. Progress in Materials Science, 46:309-327. doi: 10.1016/S0079-6425(00)00016-5 [23] Fadeev A Y, Eroshenko V A. 1997. Study of penetration of water into hydrophobized porous silicas. Journal of Colloid and Interface Science, 187:275-282. doi: 10.1006/jcis.1996.4495 [24] Falk K, Sedlmeier F, Joly L, Netz R R, Bocquet L. 2012. Ultralow liquid/solid friction in carbon nanotubes:Comprehensive theory for alcohols, Alkanes, OMCTS, and water. Langmuir, 28:14261-14272. doi: 10.1021/la3029403 [25] Ganjiani S H, Nezhad A H. 2016. Molecular dynamics simulation of the effects of the carbon-water interaction parameters on the nanofluidic energy absorption system. Journal of Physical Chemistry C, 120:11864-11870. doi: 10.1021/acs.jpcc.6b00421 [26] Goldsmith J, Martens C C. 2009. Pressure-induced water flow through model nanopores. Physical Chemistry Chemical Physics, 11:528-533. doi: 10.1039/B807823H [27] Gong X J, Li J C, Xu K, Wang J F, Yang H. 2010. A controllable molecular sieve for Na+ and K+ ions.Journal of the American Chemical Society, 132: 1873-1877. doi: 10.1021/ja905753p [28] Han A, Punyamurthula V K, Lu W Y, Qiao Y. 2008a. Deformation of a nanoporous silica under compressive loading. Journal of Applied Physics, 103:084318. doi: 10.1063/1.2909976 [29] Han A, Punyamurtula V K, Kim T, Qiao Y. 2008b. The upper limit of energy density of nanoporous materials functionalized liquid. Journal of Materials Engineering and Performance, 17:326-329. doi: 10.1007/s11665-008-9221-9 [30] Han A J, Lu W Y, Punyamurtula V K, Chen X, Surani F B, Kim T, Qiao Y. 2008a. Effective viscosity of glycerin in a nanoporous silica gel. Journal of Applied Physics, 104:124908. doi: 10.1063/1.3020535 [31] Han A J, Qiao Y. 2006. Pressure-induced infiltration of aqueous solutions of multiple promoters in a nanoporous silica. Journal of the American Chemical Society, 128:10348-10349. doi: 10.1021/ja062037a [32] Holt J K, Park H G, Wang Y M, Stadermann M, Artyukhin A B, Grigoropoulos C P, Noy A, Bakajin O. 2006. Fast mass transport through sub-2-nanometer carbon nanotubes. Science, 312:1034-1037. doi: 10.1126/science.1126298 [33] Homman A A, Bourasseau E, Stoltz G, Malfreyt P, Strafella L, Ghoufi A. 2014. Surface tension of spherical drops from surface of tension. Journal of Chemical Physics, 140:034110. doi: 10.1063/1.4862149 [34] Hoover W G. 1985. Canonical dynamics-equilibrium phase-space distributions. Physical Review A, 31:1695-1697. doi: 10.1103/PhysRevA.31.1695 [35] Hu D Y, Jiang H L, Meng K P, Xu J, Lu W Y. 2016. The impact mitigation of a heterojunction nanotube-water system:Behavior and mechanism. Physical Chemistry Chemical Physics, 18:7395-7403. doi: 10.1039/C6CP00255B [36] Hummer G, Rasaiah J C, Noworyta J P. 2001. Water conduction through the hydrophobic channel of a carbon nanotube. Nature, 414:188-190. doi: 10.1038/35102535 [37] Ibach H. 2006. Physics of Surfaces and Interfaces. New York, Springer Verlag. [38] Ismail A E, Grest G S, Stevens M J. 2006. Capillary waves at the liquid-vapor interface and the surface tension of water. Journal of Chemical Physics, 125:014702. doi: 10.1063/1.2209240 [39] Jorgensen W L, Chandrasekhar J, Madura J D, Impey R W, Klein M L. 1983. Comparison of simple potential functions for simulating liquid water. Journal of Chemical Physics, 79:926-935. doi: 10.1063/1.445869 [40] Joseph S, Aluru N R. 2008. Why are carbon nanotubes fast transporters of water? Nano Letters, 8:452-458. doi: 10.1021/nl072385q [41] Kheirabadi A M, Moosavi A. 2014. Water electrolyte transport through corrugated carbon nanopores.Physical Review E, 90:012304. http://adsabs.harvard.edu/abs/2014PhRvE..90a2304M [42] Kong X, Qiao Y. 2005a. Improvement of recoverability of a nanoporous energy absorption system by using chemical admixture. Applied Physics Letters, 86:151919. doi: 10.1063/1.1901830 [43] Kong X, Qiao Y. 2005b. Thermal effects on pressure-induced infiltration of a nanoporous system. Philo-sophical Magazine Letters, 85:331-337. doi: 10.1080/09500830500071135 [44] Kong X G, Surani F B, Qiao Y. 2005. Effects of addition of ethanol on the infiltration pressure of a mesoporous silica. Journal of Materials Research, 20:1042-1045. doi: 10.1557/JMR.2005.0140 [45] Lane L B. 1925. Freezing points of glycerol and its aqueous solutions. Industrial and Engineering Chemistry, 17:924-924. doi: 10.1021/ie50189a017 [46] Langroudi S M M, Ghassemi M, Shahabi A, Nejad H R. 2011. A molecular dynamics study of effective parameters on nano-droplet surface tension. Journal of Molecular Liquids, 161:85-90. doi: 10.1016/j.molliq.2011.04.011 [47] Lefevre B, Saugey A, Barrat J L, Bocquet L, Charlaix E, Gobin P F, Vigier G. 2004. Intrusion and extrusion of water in hydrophobic mesopores. Journal of Chemical Physics, 120:4927-4938. doi: 10.1063/1.1643728 [48] Li J C M. 2000. Damping of water infiltrated nanoporous glass. Journal of Alloys and Compounds, 310:24-28. doi: 10.1016/S0925-8388(00)01003-3 [49] Liu H L, Cao G X. 2013a. Effects of impact velocity on pressure-driven nanofluid. Journal of Chemical Physics, 139:114701. doi: 10.1063/1.4821151 [50] Liu H L, Cao G X. 2013b. Interaction between mechanical wave and nanoporous energy absorption system.Journal of Physical Chemistry C, 117:4245-4252. doi: 10.1021/jp310028x [51] Liu H L, Cao G X. 2014. Super energy absorption system based on nanofluidic glycerol solution. Journal of Physical Chemistry C, 118:25223-25233. doi: 10.1021/jp507411w [52] Liu H L, Cao G X. 2016a. Effectiveness of the Young-Laplace equation at nanoscale. Scientific Reports, 6:23936. doi: 10.1038/srep23936 [53] Liu H L, Cao G X. 2016b. Reusable energy absorption performance based on nanofluidic systems. Journal of Physical Chemistry C, 120:5213-5220. doi: 10.1021/acs.jpcc.6b00162 [54] Liu L. 2010. Nanofluidics:Fundamentals and applications in energy conversion.[PhD Thesis]. New York:Columbia University. [55] Liu L, Chen X, Kim T, Han A J, Qiao Y. 2010. Effects of anion size and concentration on electrolyte invasion into molecular-sized nanopores. New Journal of Physics, 12:033021. doi: 10.1088/1367-2630/12/3/033021 [56] Liu L, Chen X, Lu W Y, Han A J, Qiao Y. 2009a. Infiltration of electrolytes in molecular-sized nanopores.Physical Review Letters, 102:184501. doi: 10.1103/PhysRevLett.102.184501 [57] Liu L, Zhao J B, Culligan P J, Qiao Y, Chen X. 2009b. Thermally responsive fluid behaviors in hydrophobic nanopores. Langmuir, 25:11862-11868. doi: 10.1021/la901516j [58] Liu L, Zhao J B, Yin C Y, Culligan P J, Chen X. 2009c. Mechanisms of water infiltration into conical hydrophobic nanopores. Physical Chemistry Chemical Physics, 11:6520-6524. doi: 10.1039/b905641f [59] Lu Y J, Wei B. 2006. Second inflection point of water surface tension. Applied Physics Letters, 89:164106. doi: 10.1063/1.2364167 [60] Majumder M, Chopra N, Andrews R, Hinds B J. 2005. Nanoscale hydrodynamics-enhanced flow in carbon nanotubes. Nature, 438:44-44. doi: 10.1038/438044a [61] Matsumoto M, Tanaka K. 2008. Nano bubble-size dependence of surface tension and inside pressure. Fluid Dynamics Research, 40:546-553. doi: 10.1016/j.fluiddyn.2007.12.006 [62] Mo J W, Li L, Zhou J F, Xu D Y, Huang B L, Li Z G. 2015. Fluid infiltration pressure for hydrophobic nanochannels. Physical Review E, 91:033022. doi: 10.1103/PhysRevE.91.033022 [63] Nejad H R, Ghassemi M, Langroudi S M M, Shahabi A. 2011. A molecular dynamics study of nano-bubble surface tension. Molecular Simulation, 37:23-30. doi: 10.1080/08927022.2010.513007 [64] Park S H, Weng J G, Tien C L. 2001. A molecular dynamics study on surface tension of microbubbles.International Journal of Heat and Mass Transfer, 44:1849-1856. doi: 10.1016/S0017-9310(00)00244-1 [65] Punyamurtula V K, Han A, Qiao Y. 2006. An experimental investigation on a nanoporous carbon function-alized liquid damper. Philosophical Magazine Letters, 86:829-835. doi: 10.1080/09500830601064468 [66] Qiao Y, Cao G X, Chen X. 2007. Effects of gas molecules on nanofluidic behaviors. Journal of the American Chemical Society, 129:2355-2359. doi: 10.1021/ja067185f [67] Qiao Y, Liu L, Chen X. 2009. Pressurized liquid in nanopores:A modified Laplace-Young equation. Nano Letters, 9:984-988. doi: 10.1021/nl8030136 [68] Qiao Y, Punyamurtula V K, Han A J, Kong X G, Surani F B. 2006. Temperature dependence of working pressure of a nanoporous liquid spring. Applied Physics Letters, 89:251905. doi: 10.1063/1.2408664 [69] Rivera J L, Starr F W, Paricaud P, Cummings P T. 2006. Polarizable contributions to the surface tension of liquid water. Journal of Chemical Physics, 125:094712. doi: 10.1063/1.2345063 [70] Saada M A, Rigolet S, Paillaud J L, Bats N, Soulard M, Patarin J. 2010. Investigation of the energetic performance of pure silica ITQ-4 (IFR) zeolite under high pressure water intrusion. Journal of Physical Chemistry C, 114:11650-11658. doi: 10.1021/jp102663f [71] Sbragaglia M, Benzi R, Biferale L, Succi S, Toschi F. 2006. Surface roughness-hydrophobicity coupling in microchannel and nanochannel flows. Physical Review Letters, 97:204503. doi: 10.1103/PhysRevLett.97.204503 [72] Shi B, Sinha S, Dhir V K. 2006. Molecular dynamics simulation of the density and surface tension of water by particle-particle particle-mesh method. Journal of Chemical Physics, 124:204715. doi: 10.1063/1.2199849 [73] Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. 2006. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers.Proceedings of the National Academy of Sciences of the United States of America, 103:3357-3362. doi: 10.1073/pnas.0509009103 [74] Smirnov S, Vlassiouk I, Takmakov P, Rios F. 2010. Water confinement in hydrophobic nanopores. Pressure-induced wetting and drying. Acs Nano, 4:5069-5075. http://www.ncbi.nlm.nih.gov/pubmed/20690599 [75] Sodt A J, Pastor R W. 2012. The tension of a curved surface from simulation. Journal of Chemical Physics, 137:234101. doi: 10.1063/1.4769880 [76] Sun Y T, Guo Z Y, Xu J, Xu X Q, Liu C, Li Y B. 2015. A candidate of mechanical energy mitigation system:Dynamic and quasi-static behaviors and mechanisms of zeolite beta/water system. Materials & Design, 66:545-551. https://www.researchgate.net/publication/268822367_A_candidate_of_mechanical_energy_mitigation_system_Dynamic_and_quasi-static_behaviors_and_mechanisms_of_zeolite_bwater_system [77] Surani F B, Kong X G, Panchal D B, Qiao Y. 2005a. Energy absorption of a nanoporous system subjected to dynamic loadings. Applied Physics Letters, 87:163111. doi: 10.1063/1.2106002 [78] Surani F B, Kong X G, Qiao Y. 2005b. Two-staged sorption isotherm of a nanoporous energy absorption system. Applied Physics Letters, 87:251906. doi: 10.1063/1.2144280 [79] Surani F B, Qiao Y. 2006a. An energy-absorbing polyelectrolyte gel matrix composite material. Composites Part a-Applied Science and Manufacturing, 37:1554-1556. doi: 10.1016/j.compositesa.2005.11.005 [80] Surani F B, Qiao Y. 2006b. Energy absorption of a polyacrylic acid partial sodium salt-modified nanoporous system. Journal of Materials Research, 21:1327-1330. doi: 10.1557/jmr.2006.0153 [81] Surani F B, Qiao Y. 2006c. Pressure induced liquid infiltration in a functionalized poly (acrylic acid-co-acrylamide) potassium salt gel matrix material. Materials Research Innovations, 10:26-27. https://www.researchgate.net/publication/233494569_Pressure_Induced_Liquid_Infiltration_In_A_Functionalized_Polyacrylic_Acid-co_acrylamide_Potassium_Salt_Gel_Matrix_Material [82] van der Heyden F H J, Bonthuis D J, Stein D, Meyer C, Dekker C. 2007. Power generation by pressure-driven transport of ions in nanofluidic channels. Nano Letters, 7:1022-1025. doi: 10.1021/nl070194h [83] van der Heyden F H J, Stein D, Dekker C. 2005. Streaming currents in a single nanofluidic channel. Physical Review Letters, 95:116104. doi: 10.1103/PhysRevLett.95.116104 [84] Viana J C. 2006. Polymeric materials for impact and energy dissipation. Plastics Rubber and Composites, 35:260-267. doi: 10.1179/174328906X146522 [85] Walther J H, Ritos K, Cruz-Chu E R, Megaridis C M, Koumoutsakos P. 2013. Barriers to superfast water transport in carbon nanotube membranes. Nano Letters, 13:1910-1914. doi: 10.1021/nl304000k [86] Weast R C, Astle M J, Beyer W H. 1986. Handbook of Chemistry and Physics. Baca Roton:CRC Press. [87] Werder T, Walther J H, Jaffe R L, Halicioglu T, Koumoutsakos P. 2003. On the water-carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. Journal of Physical Chemistry B, 107:1345-1352. doi: 10.1021/jp0268112 [88] Wu N, Wang Q, Arash B. 2012. Ejection of DNA molecules from carbon nanotubes. Carbon, 50:4945-4952. doi: 10.1016/j.carbon.2012.06.026 [89] Wu Y B, Aluru N R. 2013. Graphitic carbon-water nonbonded interaction parameters. Journal of Physical Chemistry B, 117:8802-8813. doi: 10.1021/jp402051t [90] Xu B X, Chen X, Lu W Y, Zhao C, Qiao Y. 2014a. Non-dissipative energy capture of confined liquid in nanopores. Applied Physics Letters, 104:203107. doi: 10.1063/1.4878097 [91] Xu B X, Li Y B, Park T, Chen X. 2011. Effect of wall roughness on fluid transport resistance in nanopores.Journal of Chemical Physics, 135:144703. doi: 10.1063/1.3651158 [92] Xu B X, Qiao Y, Chen X. 2014b. Mitigating impact/blast energy via a novel nanofluidic energy capture mechanism. Journal of the Mechanics and Physics of Solids, 62:194-208. doi: 10.1016/j.jmps.2013.09.022 [93] Xu B X, Qiao Y, Zhou Q L, Chen X. 2011. Effect of electric field on liquid infiltration into hydrophobic nanopores. Langmuir, 27:6349-6357. doi: 10.1021/la200477y [94] Yu H Q, Li Y F, Li H, Zhang K, An C G, Liu X F, Liew K M. 2010. Methane molecules drive water molecules along diameter-gradient swcnts with junctions. Journal of Physical Chemistry B, 114:8676-8679. https://www.ncbi.nlm.nih.gov/pubmed/20545355 [95] Zambrano H A, Walther J H, Koumoutsakos P, Sbalzarini I F. 2009. Thermophoretic motion of water nanodroplets confined inside carbon nanotubes. Nano Letters, 9:66-71. doi: 10.1021/nl802429s [96] Zhao J B, Culligan P J, Germaine J T, Chen X. 2009. Experimental study on energy dissipation of elec-trolytes in nanopores. Langmuir, 25:12687-12696. doi: 10.1021/la901696t [97] Zhao J B, Culligan P J, Qiao Y, Zhou Q L, Li Y B, Tak M, Park T, Chen X. 2010. Electrolyte solution transport in electropolar nanotubes. Journal of Physics-Condensed Matter, 22:315301. doi: 10.1088/0953-8984/22/31/315301 [98] Zhao J B, Liu L, Culligan P J, Chen X. 2009. Thermal effect on the dynamic infiltration of water into single-walled carbon nanotubes. Physical Review E, 80:061206. doi: 10.1103/PhysRevE.80.061206 [99] Zhou Y, Dong S L. 2013. Molecular dynamics simulation of water conduction within carbon nanotube. Chinese Science Bulletin, 58:59-62. doi: 10.1007/s11434-012-5492-5 [100] Zhu F, Lu G. 2007. A review of blast and impact of metallic and sandwich structures. Electronic Journal of Structural Engineering, 7:92-101. -




 下载:
下载: